At SITC 2023, INmune Bio Inc. shared preclinical findings indicating INB03 as an innate immune checkpoint suppressor that reduces SIRPα
INmune Bio, Inc., an immunology firm in the clinical phase dedicated to developing therapies that utilize the individual's inherent immune response to combat illness, will present findings regarding the application of INB03, a dominant-negative suppressor of soluble TNF in managing high-risk MUC4 expressing HER2+ breast cancer.
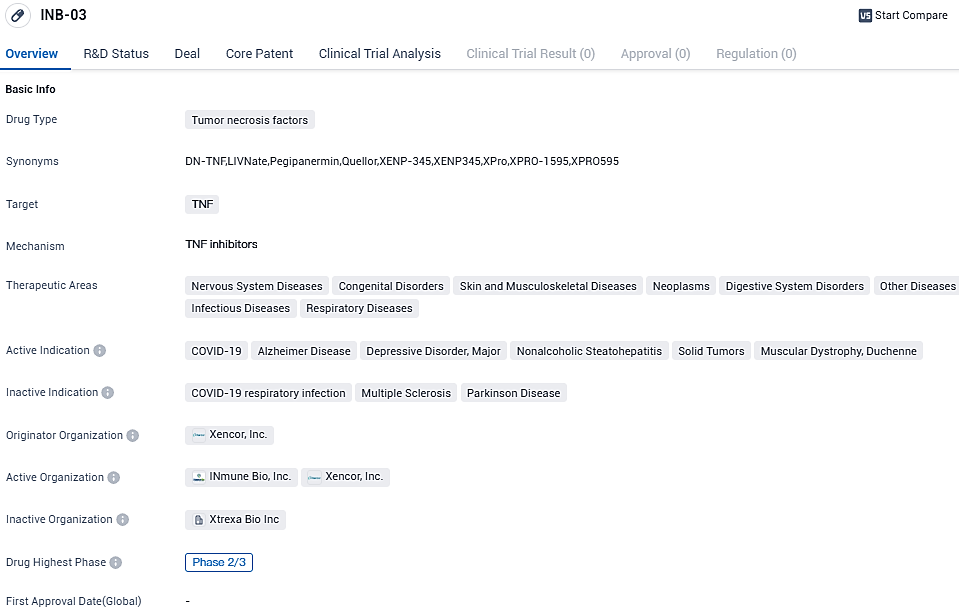
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
INB03 operates as an innate immune checkpoint suppressor that functions through the SIRPα-CD47 pathway to boost ADCP. SIRPα, a surface protein that is formed by macrophages, attaches to CD47 found in cancer cells. The SIRPα-CD47 combination serves as a "do not ingest me" signal, thwarting the phagocytosis of cancer cells and boosting resistance to immune treatment.
INB03 acts to lessen SIRPα production, negating the "do not ingest me" signal, and encouraging ADCP. When it comes to inhibiting the SIRPα-CD47 pathway, the main focus has largely been on targeting CD47 via the anti-CD47 treatment approach. Setting SIRPα as the target may yield more distinctly varied results in terms of pharmacokinetics, safety, and effectiveness.
"Macrophages hold a significant position in suppressing tumors, yet cancer cells have heeded effective strategies to escape attacks by a patient's immune system," explained Roxana Schillaci, Ph.D. of CONECIT and the lead author of the study. "TNF drives the generation of SIRPα on macrophages that stick to CD47 on cancer cells, preventing ADCP. Using INB03 to counteract sTNF decreases SIRPα creation, encouraging ADCP which should aid in maintaining tumor growth and avoiding immunotherapy resistance."
INB03 counteracts sTNF and shifts tumor-defending M2 macrophages to M1 anti-cancer macrophages, amplifies ADCP alongside trastuzumab, and downregulates SIRPα creation. In mice with trastuzumab-resistant breast cancer, administering INB03 switches splenic and tumor-penetrating macrophages to the M1 type which ingest tumor cells and lessen immune checkpoint expression (PD-L1, TIM3, LAG3) in tumor-invading CD8+ T cells.
RJ Tesi MD, CEO of INmune Bio, expressed that, "an emerging cancer treatment strategy is to enhance the efficacy of tumor-infiltrating macrophages. DN-TNF has shown significant strides in improving macrophage phagocytosis in AD, MS, DMD, and cancer experiments."
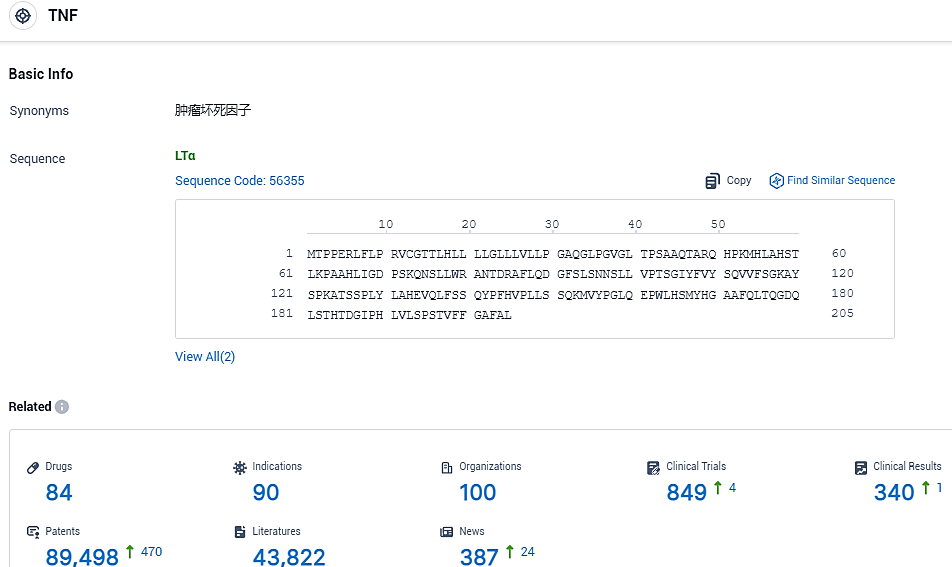
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 3, 2023, there are 84 investigational drugs for the TNF target, including 90 indications, 100 R&D institutions involved, with related clinical trials reaching 849, and as many as 89498 patents.
INB03 is a DN-TNF inhibitor that neutralizes soluble TNF without affecting transmembrane TNF or TNF receptors. INB03 decreased blood biomarkers of inflammation in patients with advanced cancer. INMB is planning a Phase II trial that uses IN03 as part of combination therapy.