B7-H3 (CD276) in Cancer Immunotherapy: Multifaceted Roles and Emerging Targeting Strategies
At the forefront of cancer treatment, scientists are continually exploring new targets and therapeutic strategies to address this global health challenge. B7-H3 (CD276), a type I transmembrane glycoprotein, has garnered significant attention due to its overexpression in a wide range of human cancers. Initially believed to function as a T-cell costimulatory molecule, B7-H3 has since been found to possess complex immunomodulatory properties, capable of both promoting and suppressing immune responses. As research has progressed, B7-H3 has been shown to play important roles not only in immune regulation but also in non-immune biological processes such as bone development and tumor progression. This multifunctionality positions B7-H3 as a highly promising novel target in oncology, spurring the development of innovative therapeutic approaches including antibody-drug conjugates (ADCs), CAR-T cell therapies, and bispecific antibodies.
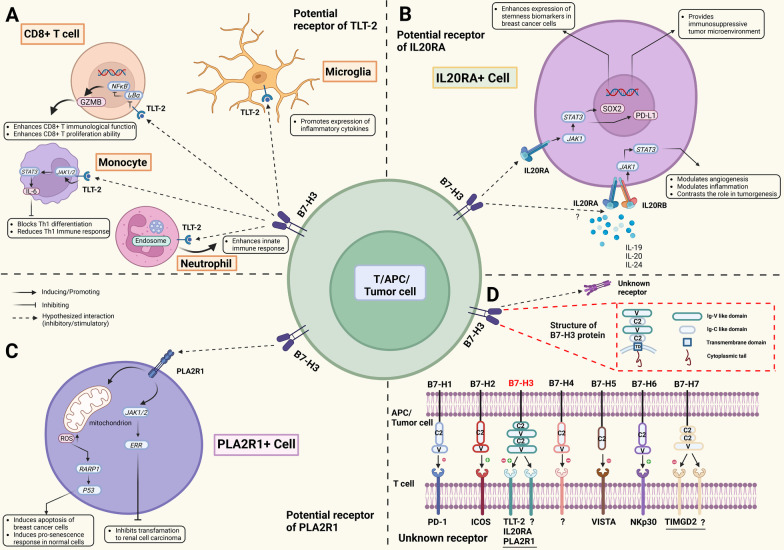
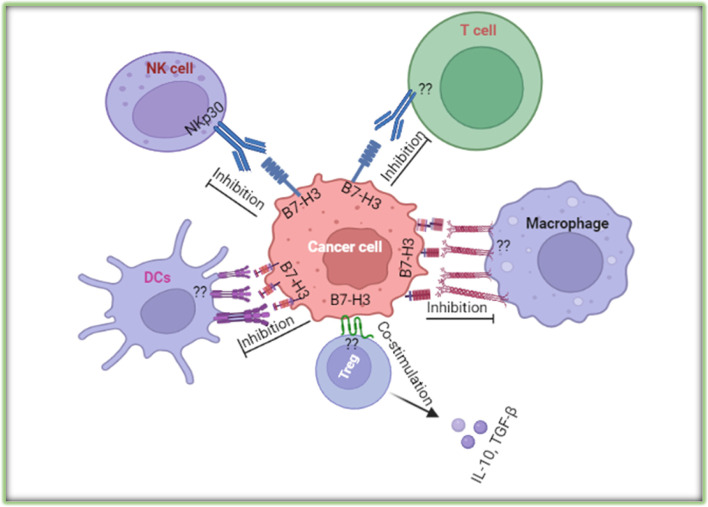
B7-H3 (CD276), as a member of the B7 superfamily, plays a multifaceted role in both immune regulation and tumor biology. It was originally identified as a T-cell costimulatory molecule due to its ability to promote the proliferation of CD4+ and CD8+ T cells and enhance the secretion of interferon-gamma (IFN-γ). This finding highlighted the molecule’s potential to amplify T-cell-mediated immune responses, contributing to the clearance of pathogens or tumor cells. Researchers observed that when B7-H3 interacts with receptors on the surface of T cells, it can significantly boost their activity, thereby enhancing the body's defense against infection and cancer.
The Coinhibitory Role of B7-H3 and Its Effects on the Immune System
While early studies focused on B7-H3’s costimulatory properties, subsequent research revealed its coinhibitory functions, particularly in dampening the activity of T cells and natural killer (NK) cells. Specifically, B7-H3 suppresses T-cell function by reducing the secretion of immune-stimulating cytokines such as interferons and tumor necrosis factor-alpha (TNF-α). It also inhibits NK cell activity, indicating that B7-H3 influences both adaptive and innate immunity. This dual role makes B7-H3 a complex immune regulator, with context-dependent biological effects that vary based on environmental and pathological conditions.
Non-Immune Functions: Bone Development and Tumor Progression
Beyond its role in the immune system, B7-H3 is also involved in non-immune processes such as regulating bone development. In tumor biology, B7-H3 has been implicated in several critical mechanisms including tumor cell proliferation, migration, angiogenesis, and epigenetic modifications. These findings suggest that B7-H3 is not merely an immune checkpoint molecule but also a key player in maintaining physiological balance and driving disease progression. Notably, high levels of B7-H3 expression in malignant tumors are often associated with increased invasiveness and metastatic potential, underscoring its clinical relevance in oncology.
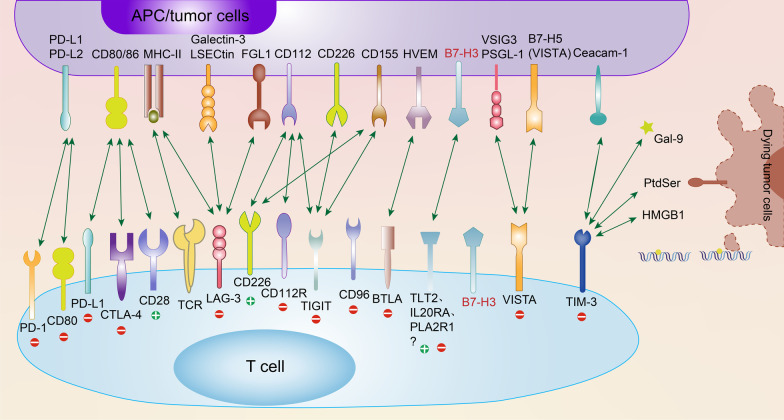
B7-H3 as a Novel Target in Cancer Immunotherapy
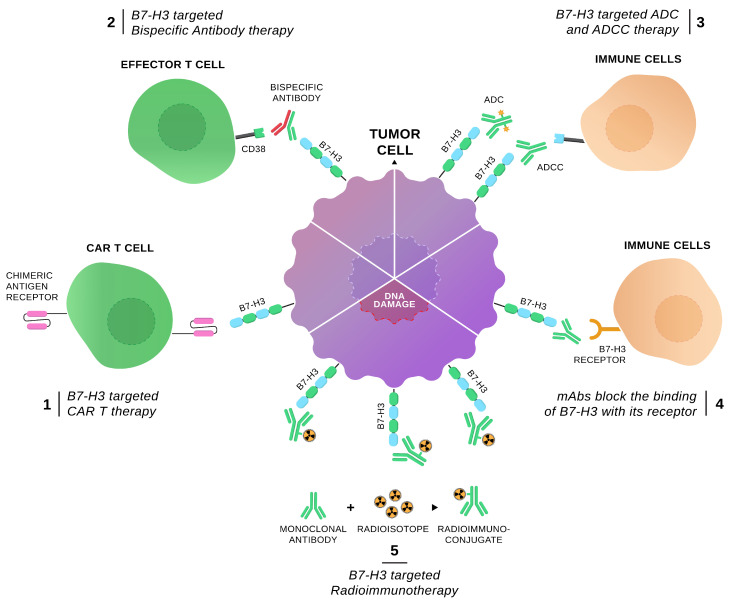
Given its widespread overexpression in various cancers and its role in facilitating immune evasion, B7-H3 has emerged as a promising target for cancer immunotherapy. Multiple therapeutic strategies are currently under development to target B7-H3, including antibody-drug conjugates (ADCs), CAR-T cell therapies, and bispecific antibodies. These approaches aim to block the immunosuppressive functions of B7-H3 and restore or enhance the immune system's ability to effectively attack tumor cells, offering new hope and treatment options for cancer patients.
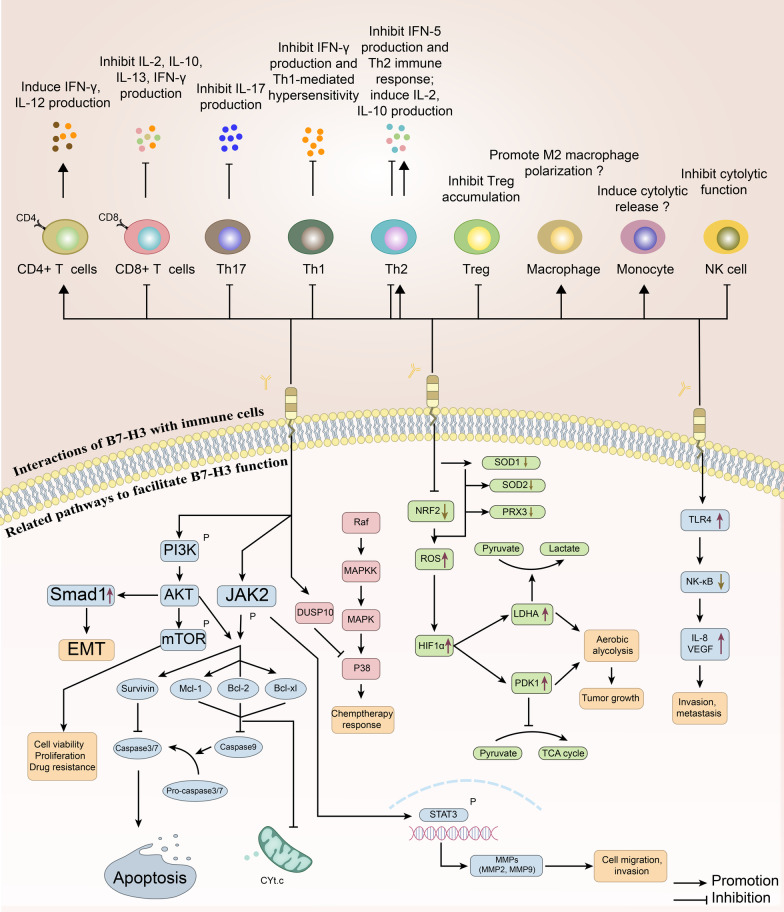
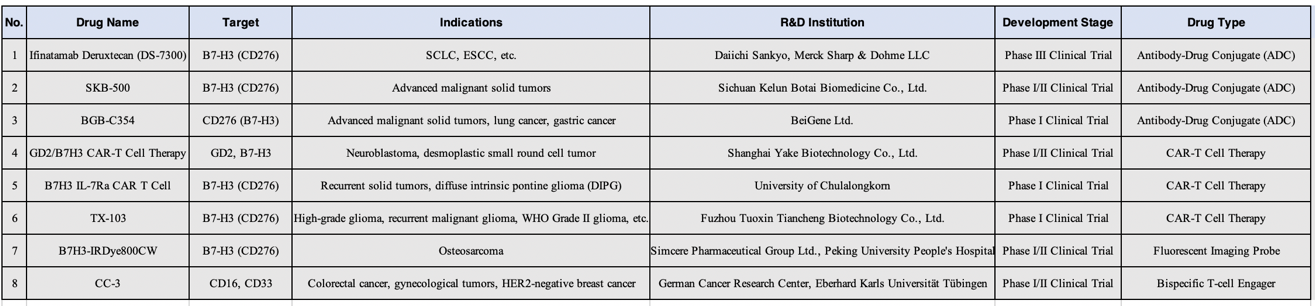
I. Antibody-Drug Conjugates (ADCs) Targeting B7-H3
Ifinatamab Deruxtecan (DS-7300, DS-7300a), BGB-C354, and SKB-500 are all antibody-drug conjugates (ADCs) targeting the B7-H3 (CD276) antigen, but they differ in terms of development progress, mechanism of action, and technical characteristics.
1) Ifinatamab Deruxtecan
Ifinatamab Deruxtecan (DS-7300, DS-7300a) is an antibody-drug conjugate (ADC) designed to leverage the high specificity of monoclonal antibodies to target antigens expressed on the surface of tumor cells. By selectively binding to B7-H3, Ifinatamab Deruxtecan delivers a cytotoxic payload directly to cancer cells, thereby minimizing damage to healthy tissues.
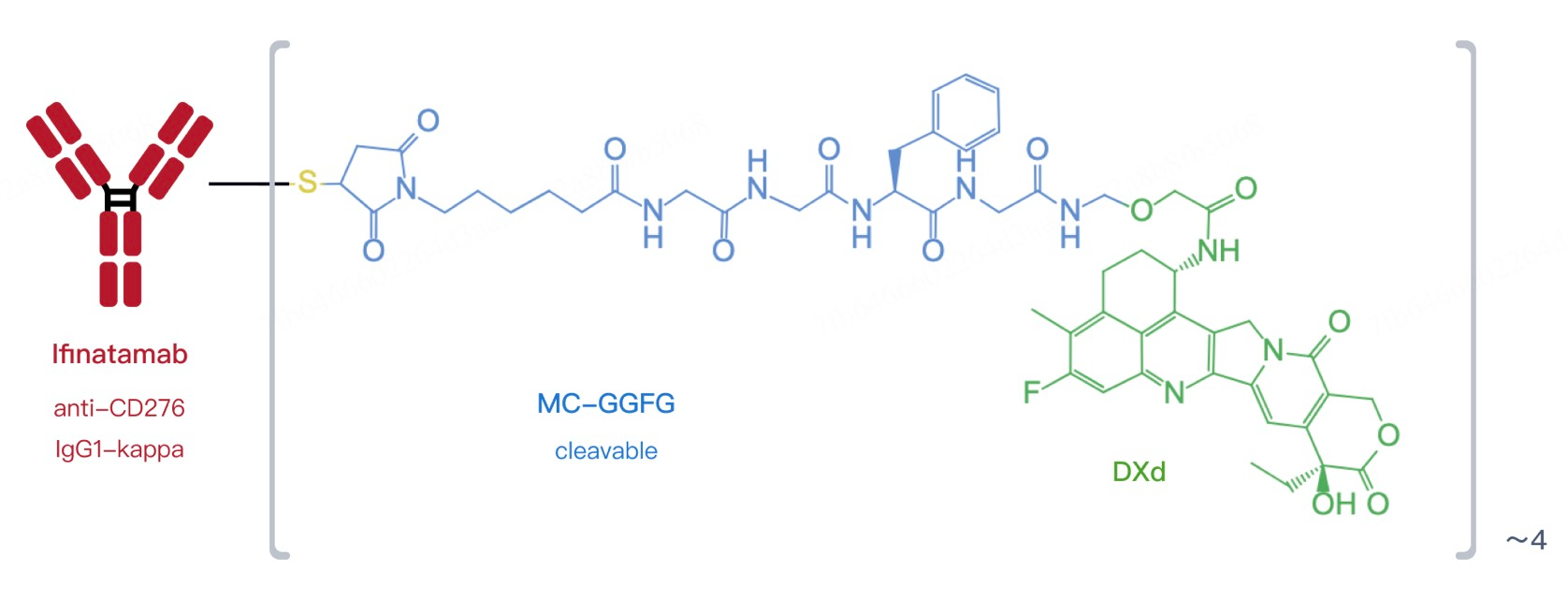
This ADC consists of a humanized anti-B7-H3 IgG1 monoclonal antibody linked to the topoisomerase I inhibitor DXd. Upon binding to B7-H3 on the cancer cell surface, the entire complex is internalized. Inside the cell, the linker is enzymatically cleaved, releasing DXd. As a potent cytotoxic agent, DXd disrupts DNA structure within cancer cells, inducing apoptosis. This approach enhances treatment precision and helps overcome drug resistance.
As of the latest data, Ifinatamab Deruxtecan has entered global Phase III clinical trials—a significant milestone in its development. In the earlier Phase II IDeate-Lung01 study, it demonstrated promising results, particularly in patients with extensive-stage small cell lung cancer (ES-SCLC), achieving an objective response rate (ORR) of 54.8%. The drug has also shown potential in other cancer types.
Given the widespread overexpression of B7-H3 across various malignancies, Ifinatamab Deruxtecan may benefit a broad range of cancers beyond a single type, including diseases of the digestive and respiratory systems. Further research may expand its indications, positioning it as a key weapon against refractory tumors.
While Ifinatamab Deruxtecan has shown significant efficacy, it is also associated with some risks. Common adverse events include nausea, anemia, and fatigue. To ensure optimal safety and efficacy, researchers are closely monitoring side effects and exploring optimized dosing strategies to reduce patient burden.
2) BGB-C354
BGB-C354 operates through a similar mechanism, using a monoclonal antibody specific for B7-H3 conjugated with a topoisomerase I inhibitor (TOP1). When the ADC binds to B7-H3-expressing tumor cells, the complex is internalized, and the linker is cleaved to release the cytotoxic payload, which damages the DNA of the cancer cell, leading to its death. This enhances treatment specificity while reducing harm to surrounding healthy tissue.
BGB-C354 is currently undergoing Phase I clinical trials to assess its safety, tolerability, pharmacokinetics, and preliminary antitumor activity. This phase is critical for establishing the optimal dose range and supporting future Phase II and III studies. Notably, BGB-C354 has shown potential applications in patients with advanced solid tumors, including lung and gastric cancers.
Compared to Daiichi Sankyo’s DS-7300, BGB-C354 has a higher drug-to-antibody ratio (DAR) of 8, versus 4 for DS-7300. A higher DAR means each antibody molecule carries more cytotoxic payload, which could enhance tumor-killing efficacy. However, this also requires careful design to balance potency with tolerability and avoid unacceptable toxicity.
3) SKB-500
SKB-500 is designed to deliver a cytotoxic drug directly into cancer cells, minimizing harm to healthy tissues. It uses a high-affinity humanized monoclonal antibody to recognize and bind B7-H3 on tumor cells. The other end of the antibody is linked to a potent cytotoxic agent. Upon successful binding, the entire complex is internalized, and the cytotoxic payload is released inside the cell to destroy key structures such as DNA or microtubules, leading to cancer cell death. This strategy improves treatment precision and helps combat drug resistance.
SKB-500 is currently in Phase I/II clinical trials to evaluate its safety, tolerability, and preliminary efficacy. These early studies are essential for determining optimal dosing and laying the foundation for broader clinical development. Researchers are also exploring its use across a range of advanced solid tumors, including but not limited to lung cancer and gastric cancer.
Because B7-H3 is widely expressed in many human cancers, SKB-500 has broad therapeutic potential. In addition to lung and gastric cancers, it may also prove effective in treating other advanced solid tumors such as breast and prostate cancers. As more clinical data emerges, SKB-500 may offer a new treatment option for patients who do not respond well to conventional therapies.
II. CAR T Therapies Targeting B7-H3
1) B7H3 IL-7Ra CAR T Cell Therapy
B7H3 IL-7Ra CAR T cell therapy is an innovative autologous CAR T cell approach specifically designed to target B7-H3 (CD276). By reprogramming a patient’s T cells into "smart bombs" that specifically attack B7-H3–positive cancer cells, this therapy offers a more precise and effective treatment option. The core mechanism involves genetically engineering T cells to express a chimeric antigen receptor (CAR) on their surface that can recognize and bind to B7-H3 on tumor cells. To enhance the persistence and anti-tumor activity of these engineered T cells, an IL-7Ra signaling domain is incorporated into the CAR construct. This modification helps counteract immunosuppressive effects within the tumor microenvironment and promotes prolonged in vivo survival and function of the T cells. As a result, B7H3 IL-7Ra CAR T cells not only kill tumor cells directly but also help recruit other immune cells to support the anti-cancer response.
This therapy is currently in Phase I clinical trials, marking its transition from preclinical research to human testing. The primary goals of this phase are to evaluate safety, tolerability, and to gather preliminary efficacy data. While early results have not yet been published, the therapy has shown potential in small patient populations, especially in cases of relapsed solid tumors and diffuse intrinsic pontine glioma (DIPG), which typically respond poorly to conventional therapies.
DIPG is located deep within the brain and is difficult to surgically remove, making standard radiation and chemotherapy largely ineffective. B7H3 IL-7Ra CAR T therapy may offer new hope for such patients. Given the widespread expression of B7-H3 across multiple cancer types, this technology could potentially be expanded to treat a broader range of malignancies.
Despite its promise, CAR T therapy carries inherent risks and challenges. Common adverse events include cytokine release syndrome (CRS) and neurotoxicity. Therefore, close patient monitoring and prompt adjustments to the treatment plan are critical during early-phase trials. Researchers are also working to optimize CAR constructs and manufacturing processes to further enhance safety and efficacy.
2) GD2/B7H3 CAR T Cell Therapy
GD2/B7H3 CAR T cell therapy is a novel immunotherapy that targets two antigens—disialoganglioside (GD2) and B7-H3 (CD276)—to enhance tumor cell recognition and killing. Both antigens are highly expressed in various cancers, particularly in pediatric neuroblastoma. This dual-targeting strategy improves the specificity of CAR T therapy and may help overcome resistance and relapse often seen with single-antigen targeting.

In this design, CAR T cells are engineered to recognize and bind both GD2 and B7H3, enabling more effective detection and destruction of cancer cells. Notably, researchers have implemented a SynNotch system, which acts as a logic gate: CAR T cells are first primed by GD2 recognition, and only upon encountering sufficient B7-H3 in the tumor microenvironment is their CAR function activated. This gatekeeping mechanism enhances treatment precision, reduces potential off-target effects, and improves T cell persistence and activity in vivo.
According to recent data, GD2/B7H3 CAR T therapy has demonstrated significant efficacy in preclinical models. For example, in a mouse model of metastatic neuroblastoma, this dual-target CAR T cell therapy not only effectively controlled tumor growth but also showed favorable metabolic adaptation and reduced T cell exhaustion. These findings lay a solid foundation for future clinical trials and support continued development.
Although initially focused on neuroblastoma, GD2/B7H3 CAR T therapy may be applicable to a wide range of solid tumors due to the broad expression of both GD2 and B7-H3. This approach may be especially beneficial for patients who have not responded well to traditional therapies or who have developed drug resistance. As more clinical data become available, the therapy could be expanded to include adult patients and a wider range of cancers.
3) TX-103
TX-103 is another autologous CAR T cell therapy. It involves engineering a patient’s T cells to express a chimeric antigen receptor that specifically recognizes the B7-H3 molecule. Once re-infused into the patient, these engineered T cells can precisely locate and attack B7-H3–expressing tumor cells. This approach enhances treatment specificity and may reduce damage to healthy tissue, thereby minimizing side effects. Because B7-H3 is expressed in many cancer types, TX-103 has a wide range of potential applications—from brain tumors to other advanced solid tumors.
TX-103 is currently undergoing Phase I clinical trials, primarily to assess its safety and feasibility in patients with relapsed malignant gliomas and other B7-H3–positive solid tumors. In addition to monitoring the incidence and severity of adverse events, researchers aim to determine the maximum tolerated dose and establish appropriate dosing regimens for subsequent Phase II studies. Secondary endpoints include evaluating treatment response rates, monitoring cytokine level fluctuations, and analyzing changes in immune cell phenotypes. These data are essential for understanding TX-103's mechanism of action and long-term efficacy.
III. Bispecific T Cell Engagers Targeting B7-H3
CC-3 is a bispecific antibody (bsAb) developed by the German Cancer Consortium (DKTK), engineered with dual specificity for CD276 (B7-H3) and CD3. It is being developed to treat patients with metastatic colorectal cancer (CRC) who have failed at least three prior lines of therapy. What sets CC-3 apart is its dual-targeting mechanism—simultaneously binding to CD276 on tumor cells and tumor-associated vasculature—enabling a two-pronged attack on both cancer cells and their blood supply. By engaging CD3, CC-3 directs T cells to target and destroy CD276-expressing tumor cells and enhances immune cell infiltration into the tumor microenvironment.
CC-3 exerts its antitumor effect through a bispecific antibody design. One arm binds specifically to CD3 on T cells, activating them even in the absence of conventional T cell receptor (TCR)-mediated antigen recognition. Once activated, T cells release cytotoxic molecules such as perforin and granzyme to kill target cells. The other arm of CC-3 binds to CD276 on the surface of tumor cells, guiding T cells to CD276-positive cells and mediating their destruction.
In addition to direct tumor cell targeting, CC-3 interacts with tumor vasculature in CRC, helping to disrupt the tumor’s blood supply and "starve" it, while also facilitating immune cell access to the tumor. CC-3 was specifically engineered to minimize off-target effects by promoting T cell activation primarily within the tumor microenvironment rather than systemically, thereby reducing the risk of adverse effects.
To enhance in vivo stability, CC-3 incorporates YTE modifications—engineered mutations that extend the antibody’s half-life in human serum. This prolongs drug activity, simplifies dosing regimens, and improves patient convenience and compliance.
The first-in-human clinical trial for CC-3 includes two phases: an initial dose-escalation phase to determine the maximum tolerated dose (MTD), followed by a dose-expansion phase aimed at identifying the recommended Phase II dose. These studies will also evaluate CC-3’s pharmacokinetics, including its half-life and immunogenicity, and assess immune responses induced by the therapy. Given the differences between animal models and humans—particularly in pharmacokinetics and immunogenicity—conducting these evaluations in humans is critical.
As a novel treatment strategy, CC-3 holds promise for patients with metastatic CRC who are unresponsive to or have relapsed after standard therapies. More importantly, since CD276 is expressed on both tumor cells and vasculature, the application of CC-3 could extend beyond CRC to other types of solid tumors. Thus, CC-3 not only offers a potential breakthrough for treatment-resistant cancers but also opens new avenues for broadening B7-H3–targeted therapies.
Conclusion
In summary, B7-H3 has emerged as a compelling target in cancer therapy due to its high expression across various malignancies. Initially identified as a co-stimulatory molecule, B7-H3 was later found to have co-inhibitory functions and roles in non-immune biological processes, underscoring its complex, multifaceted nature.
Innovative therapies targeting B7-H3—including antibody-drug conjugates (ADCs) like Ifinatamab Deruxtecan, BGB-C354, and SKB-500, as well as CAR T cell therapies like B7H3 IL-7Ra CAR T and GD2/B7H3 CAR T cells—have shown promising anticancer potential. These therapies precisely target tumor cells and enhance immune responses, offering new hope for patients with treatment-resistant cancers.
Despite ongoing challenges such as managing side effects and optimizing efficacy, advances in research and technology continue to position B7-H3 as a key component of future precision medicine, heralding a new era in cancer treatment. Meanwhile, bispecific antibodies like CC-3 further expand the therapeutic landscape, signaling a shift toward more precise and effective immunotherapies in oncology.
How to obtain the latest development progress of all targets?
In the Synapse database, you can stay updated on the latest research and development advances of all targets. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1.Zhao B, Li H, Xia Y, Wang Y, Wang Y, Shi Y, Xing H, Qu T, Wang Y, Ma W. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022 Oct 25;15(1):153. doi: 10.1186/s13045-022-01364-7. PMID: 36284349; PMCID: PMC9597993.
- 2.Getu AA, Tigabu A, Zhou M, Lu J, Fodstad Ø, Tan M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol Cancer. 2023 Mar 2;22(1):43. doi: 10.1186/s12943-023-01751-9. PMID: 36859240; PMCID: PMC9979440.
- 3.Lin YJ, Mashouf LA, Lim M. CAR T Cell Therapy in Primary Brain Tumors: Current Investigations and the Future. Front Immunol. 2022 Feb 21;13:817296. doi: 10.3389/fimmu.2022.817296. PMID: 35265074; PMCID: PMC8899093.
- 4.Koumprentziotis IA, Theocharopoulos C, Foteinou D, Angeli E, Anastasopoulou A, Gogas H, Ziogas DC. New Emerging Targets in Cancer Immunotherapy: The Role of B7-H3. Vaccines (Basel). 2024 Jan 5;12(1):54. doi: 10.3390/vaccines12010054. PMID: 38250867; PMCID: PMC10820813.