May 2025 Patent Highlights: Roche's tri-complex inhibitors
F.Hoffmann-La Roche(Roche) disclosed two patent applications, WO2025093625A1 and WO2025104149A1, covering a series of novel macrocyclic KRAS G12C inhibitors for the treatment or prevention of KRAS mutation-driven cancers, including pancreatic adenocarcinoma, colorectal cancer, and non-small cell lung cancer. The compounds disclosed in patent WO2025093625A1 appear to be analogs of Zoldonrasib (RMC-9805) developed targeting the KRAS G12D signaling pathway, while those from WO2025104149A1 are highly similar to Elironrasib, with the compound (Example 1) differing from Elironrasib by only a single fluorine atom.
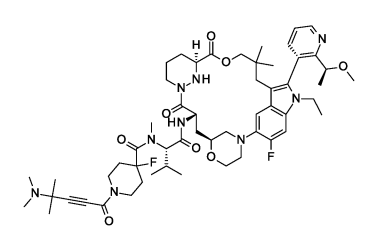
Example 1(WO2025104149A1)
NCI-H358 G12C IC50 (nM):0.42
30 mg/kg, po Cmax(ng/mL):9919;AUCo-last(hxng/mL):24959
NCI-H2122 xenograft TGI:108%(Day21)
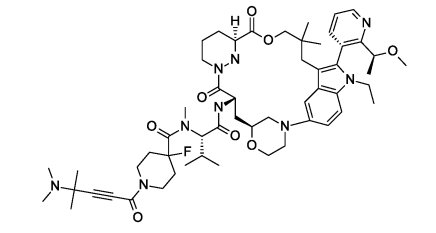
Elironrasib (RMC-6291)
NCI-H358 CTG IC50 (nM):0.09(AACR2023)
30 mg/kg, po Cmax(ng/mL):2706;AUCo-last(hxng/mL):4116
NCI-H2122 xenograft TGI:92%(Day21) (WO2025104149A1)
Mutations in the Kirsten rat sarcoma viral oncogene homologue (KRAS) gene are the most common oncogenic driver mutations found in human cancer.The glycine-to-cysteine mutation at position 12 (G12C) of the KRAS protein interferes with guanosine triphosphate (GTP) hydrolysis, thus keeping KRAS primarily in the active, GTP-bound state. The increased activity of the KRAS G12C protein drives oncogenic signaling through multiple downstream pathways that directly promote tumor-cell survival, proliferation, and metastasis.
Roche is currently developing a covalent KRAS G12C inhibitor, Divarasib (GDC-6036), which irreversibly locks the protein in an inactive state by binding to a cysteine residue, thereby blocking its oncogenic signaling. Tri-complex inhibitors, on the other hand, are RAS(ON) inhibitors that selectively target the active state of RAS.Therefore, the combination of these two mechanisms in clinical treatment may offer the potential for synergistic effects.In a Phase I clinical trial, Divarasib (GDC-6036) demonstrated an objective response rate (ORR) of 56.4% in advanced non-small cell lung cancer (NSCLC), comparable to Elironrasib’s ORR of 56%, but with a longer median progression-free survival (mPFS).
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!