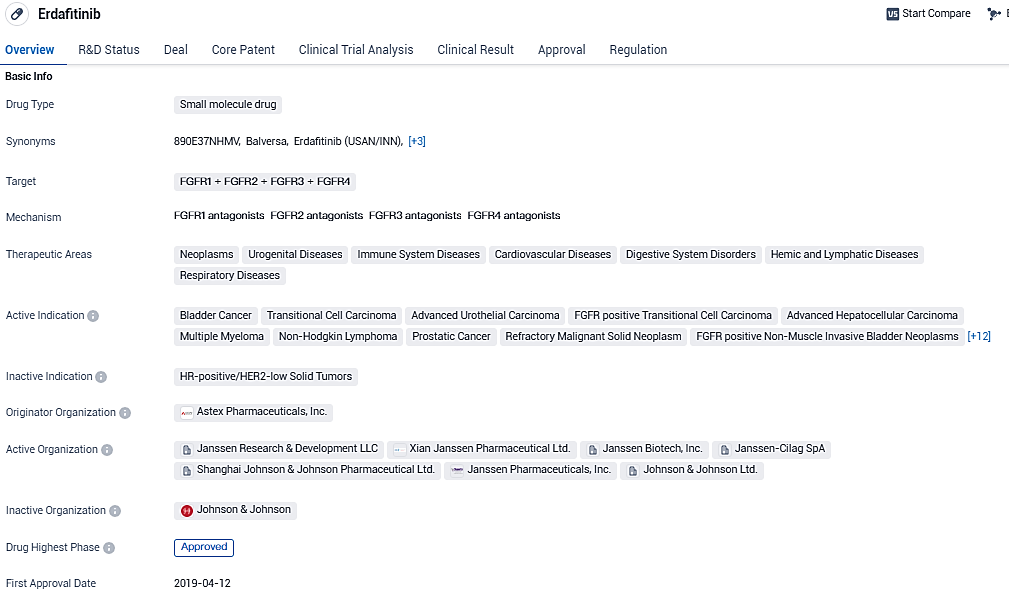
BALVERSA® approved by the U.S. FDA to treat advanced or metastatic bladder cancer with genetic mutations
Johnson & Johnson disclosed that the U.S. Food and Drug Administration has sanctioned a supplementary New Drug Application for BALVERSA® (erdafitinib) intended for the management of adult patients diagnosed with either locally advanced or metastatic urothelial carcinoma. This approval targets patients showing susceptible gene alterations in fibroblast growth factor receptor 3 (FGFR3) and who have experienced disease progression following at least one round of prior systemic therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
BALVERSA® is not suggested for patients who qualify for, but have not undergone prior therapy with PD-1 or PD-L1 inhibitors. The FDA's move effectively changes the accelerated approval of BALVERSA® in April 2019 to full approval, thanks to the clinical and overall survival advantages observed in the Phase 3 THOR study. BALVERSA® is the premier oral FGFR kinase inhibitor to get approval and the only targeted treatment option for patients with mUC and FGFR alterations.
Around 20% of mUC patients possess FGFR3 gene alterations. After undergoing at least one line of systemic therapy, which includes a checkpoint inhibitor, such patients often face a dismal prognosis with limited available treatment methods. The approval is based on outcomes from Cohort 1 of the randomized, supervisory, open-label, multicenter Phase 3 THOR study that verified BALVERSA®’s clinical advantage in prolonging overall survival compared to second-line chemotherapy.
The study’s results revealed a 36% reduction in death risk with BALVERSA® versus chemotherapy in patients who had undergone previous treatment with PD-1 or PD-(L)1 inhibitor. Those treated with BALVERSA® lived an average of more than four months longer.
"Randomized Phase 3 data continue to evidence the potential of BALVERSA in the treatment of patients with advanced bladder cancer," expressed Kiran Patel, MD, Vice President, Clinical Development, Solid Tumors, Johnson & Johnson Innovative Medicine. "This significant development fortifies our dedication to advancing innovative, precision therapies in oncology and affirming the role of targeted therapy in bladder cancer treatment."
Johnson & Johnson is supplying BALVERSA® and related patient services through a single-source specialty pharmacy provider, US Bioservices. This approach aligns with the company's ongoing pledge to present high-quality products, services, accessibility, and aid to healthcare professionals and patients.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
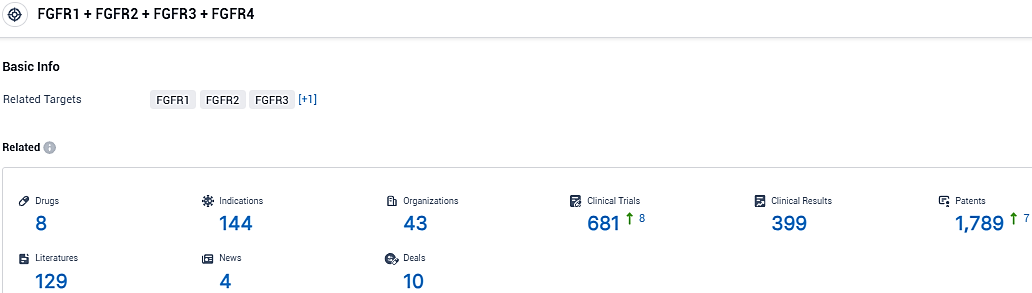
According to the data provided by the Synapse Database, As of January 25, 2024, there are 8 investigational drugs for the FGFR1 and FGFR2 and FGFR3 and FGFR4 target, including 144 indications, 43 R&D institutions involved, with related clinical trials reaching 681, and as many as 1789 patents.
BALVERSA® received Breakthrough Therapy Designation from the U.S. FDA in 2018 and received accelerated approval in 2019 for the treatment of adults with locally advanced or mUC which has susceptible FGFR3 or FGFR2 genetic alterations and who have progressed during or following at least one line of prior platinum-containing chemotherapy, including within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy.