Novartis Lutathera® cuts risk of progression or death in gastroenteropancreatic neuroendocrine tumors by 72%
Novartis delivered findings from the Phase III NETTER-2 study, indicating that Lutathera (INN: lutetium (177Lu) oxodotreotide / USAN: lutetium Lu 177 dotatate) coupled with extended-release octreotide, decreased the threat of disease advancement or fatality by 72%. This was used as an initial treatment regiment for patients suffering from somatostatin receptor-positive (SSTR+) advanced gastroenteropancreatic neuroendocrine tumors of well-differentiated grade 2/3, as opposed to the use of high-dose octreotide LAR alone.
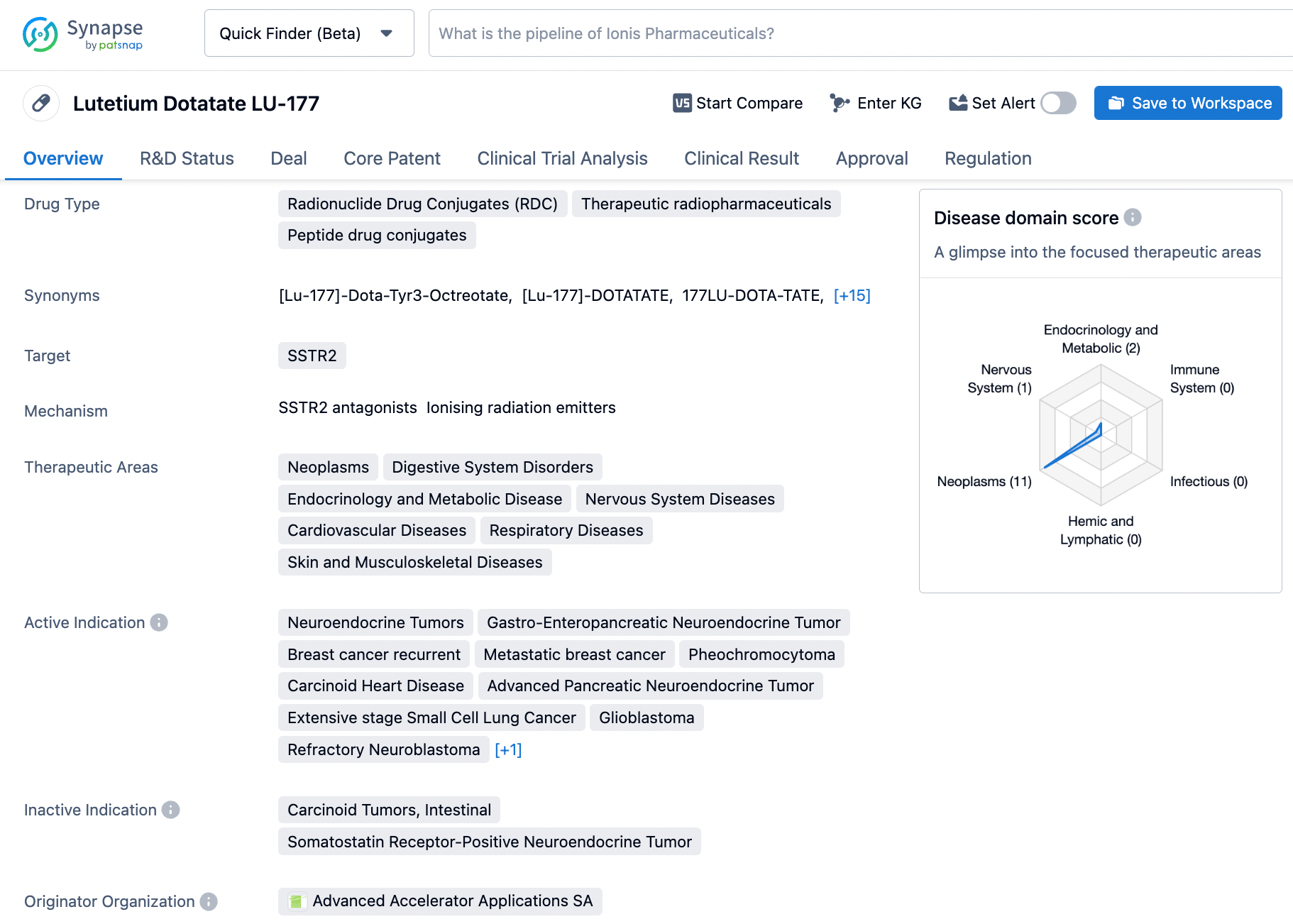
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Lutathera shows promising results that could shift present practices and proposes novel first-line therapeutic data for patients with a critical unaddressed requirement. The study supports the clinical advantage of utilizing radioligand therapy as a primary solution for newly diagnosed patients suffering complex GEP-NETs," expressed Dr. Simron Singh, associate professor of medicine at the University of Toronto and co-founder of the Susan Leslie Clinic for Neuroendocrine Tumours at the Odette Cancer Centre, "The gathered evidence will encourage other professionals to consider using Lutathera as the primary recourse for patients battling against this fatal cancer variant."
"Initiating a successful Phase III trial for a radioligand therapy in a first-line setting is unprecedented. The comprehensive efficacy and secure outcomes coincide with the high relevance found in this variety of developed cancer, addressing the urgent need for treatment in freshly diagnosed advance GEP-NET patients," commented Jeff Legos, Global Head of Oncology Development at Novartis.
"The promising outcomes represent an essential progress and reasserts our tactical study and creation of radioligand therapies in earlier treatment lines or disorder phases to enhance patient outcomes," added Legos.
NETs are cancers originating from neuroendocrine cells across the body, typically viewed as slow-progressing malignancies. Nonetheless, specific NETs are coupled with quick progression and a poor forecast, often leaving diagnosis until patients reach the progressed disease state. Even though NETs are classified as uncommon, the occurrences have spiked in the past decades and thus emphasis on discovering treatment possibilities for newly diagnosed cases perpetuates.
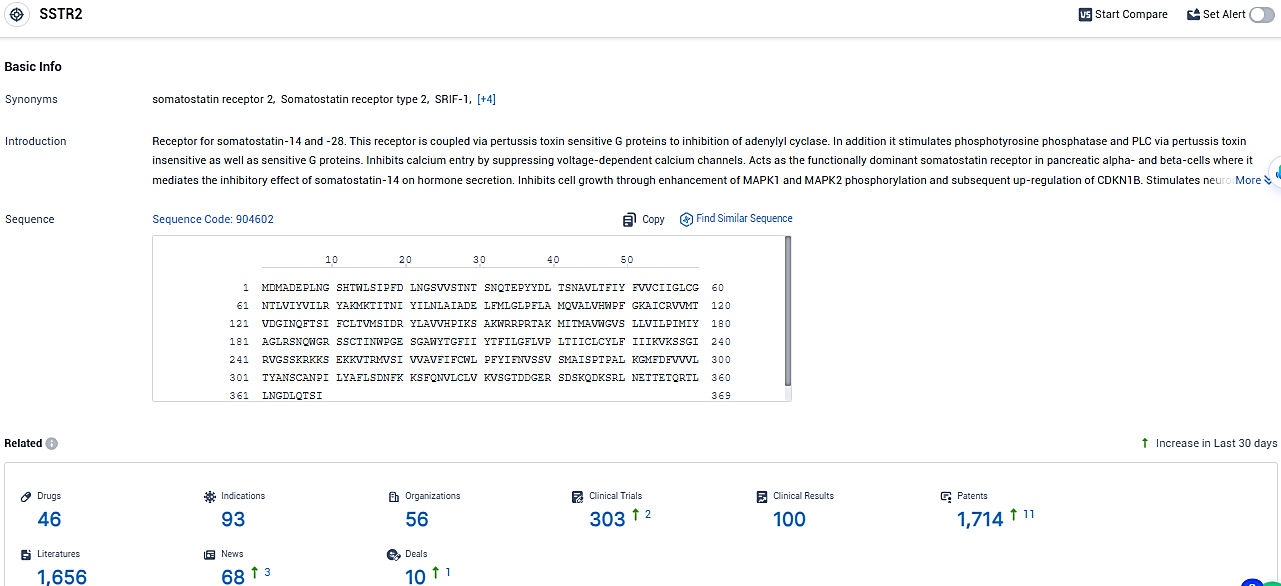
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 25, 2024, there are 46 investigational drugs for the SSTR2 target, including 93 indications, 56 R&D institutions involved, with related clinical trials reaching 303, and as many as 1714 patents.
Lutathera is approved in the US for the treatment of adult patients with SSTR-positive GEP-NETs, including those in the foregut, midgut and hindgut, an indication which includes the NETTER-2 population. Lutathera is also approved in Europe for unresectable or metastatic, progressive, well-differentiated, SSTR-positive GEP-NETs in adults®7,8, and in Japan for SSTR-positive NETs.