Basking Biosciences Begins Phase 2 Trial of BB-031 in Acute Ischemic Stroke Patients
Basking Biosciences (Basking), a biopharmaceutical company in the clinical development phase focused on innovative acute thrombolytic treatments, has reported that the initial group of patients have received doses in the RAISE Phase 2 clinical study. This trial is assessing the efficacy of BB-031 in patients experiencing acute ischemic stroke (AIS).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are pleased to announce the commencement of patient enrollment for the RAISE trial," stated Richard Shea, Chief Executive Officer of Basking. "This achievement moves us closer to providing a next-generation therapy that we believe will offer patients an alternative thrombolytic option to conventional fibrinolytics."
RAISE is a two-part, Phase 2 clinical study aimed at assessing the safety, tolerability, preliminary efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of BB-031 in 156 patients experiencing acute ischemic stroke within 24 hours of onset. This trial is a multicenter, double-blind, placebo-controlled, randomized single ascending dose study. Further details can be found on clinicaltrials.gov.
"Safely and effectively targeting the recanalization of acutely blocked intracranial arteries using innovative pharmacology has the potential to increase treatment availability for stroke patients and address significant unmet needs in acute stroke care," said Michael Hill, M.D., Professor at the University of Calgary and Foothills Medical Centre, and Clinical Program Advisor and Principal Investigator of RAISE. "Many patients are ineligible for existing drug treatments or cannot access a comprehensive stroke center in time to benefit from advanced endovascular therapy."
"We are excited to get this study running – patients are in anticipation," noted Shahid M. Nimjee, M.D., Ph.D., co-founder, Chief Medical Officer of Basking, and Professor of Neurosurgery and Surgical Director of the Comprehensive Stroke Center at The Ohio State University Wexner Medical Center. "We believe BB-031 will significantly broaden the treatment options for stroke patients. Recanalization could be made safer with our reversal agent, BB-025, which is expected to enter clinical development next year. Preclinical studies have demonstrated BB-025’s capacity to neutralize BB-031 within minutes."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
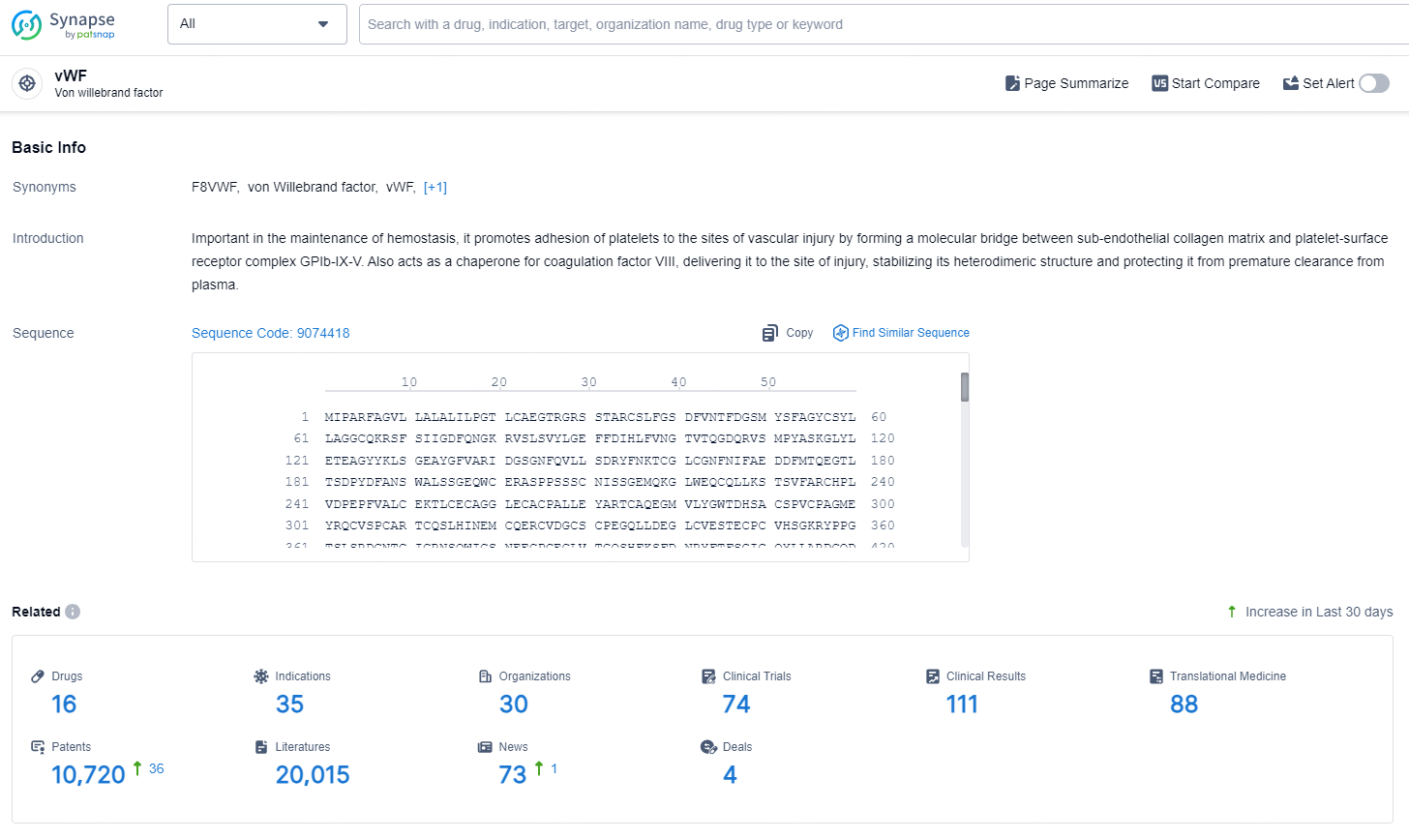
According to the data provided by the Synapse Database, As of September 11, 2024, there are 16 investigational drugs for the vWF targets, including 35 indications, 30 R&D institutions involved, with related clinical trials reaching 74, and as many as 10720 patents.
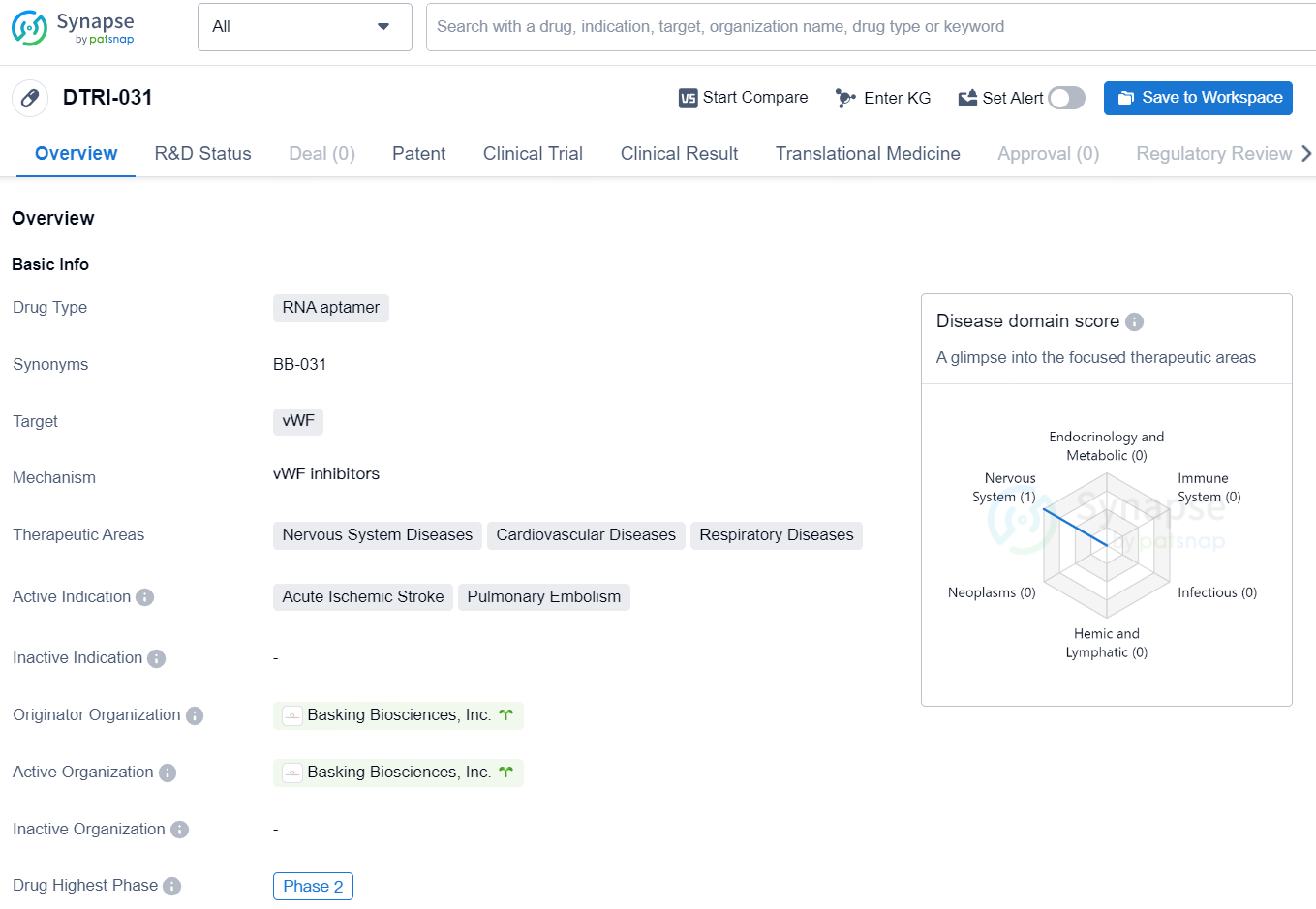
The drug DTRI-031 is an RNA aptamer designed to target the von Willebrand Factor (vWF). It is primarily focused on addressing diseases related to the nervous system, cardiovascular system, and respiratory system. The active indications for DTRI-031 include acute ischemic stroke and pulmonary embolism. The drug is in the Phase 2 stage of development and is being developed by Basking Biosciences, Inc.