Unveiling the Development History and Market Prospects of Lenacapavir
The global HIV drug market is highly competitive, with multiple pharmaceutical companies collaborating in research and commercialization to provide more effective treatment options. Gilead Sciences and Merck & Co. are among the main players in this field. They collaborated to develop long-acting HIV treatment regimens, including long-acting oral and injectable formulations, which combine Gilead's capsid inhibitor lenacapavir and Merck's nucleoside reverse transcriptase translocation inhibitor islatravir.
Lenacapavir by Gilead is a potential "first-in-class" capsid inhibitor, which received breakthrough therapy designation from the U.S. FDA in 2019 for the treatment of heavily treatment-experienced patients with multidrug-resistant HIV-1 infection. The advantage of this drug lies in its potential to maintain efficacy with an injection only once every six months.
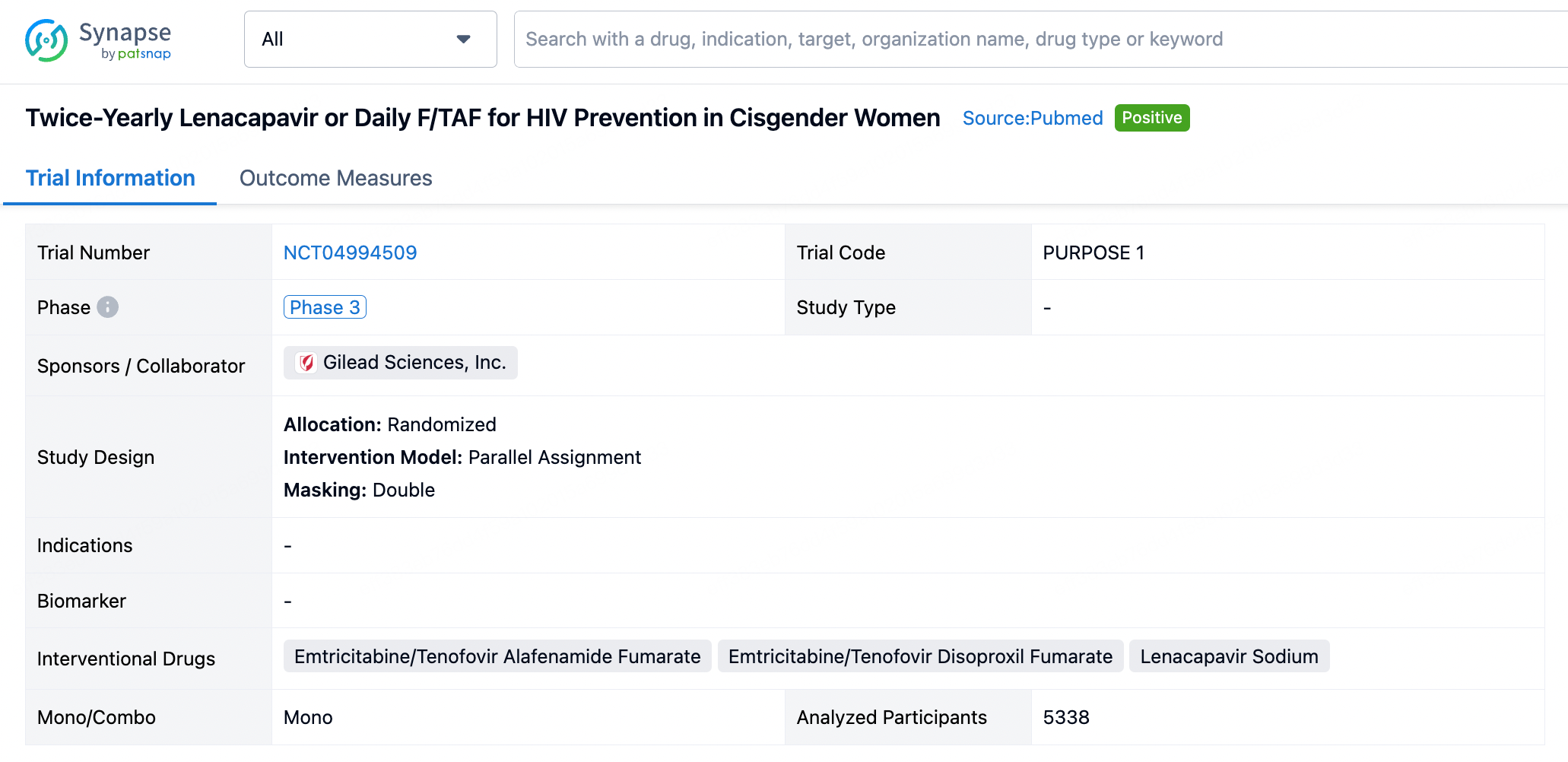
In July of this year, Gilead announced results from a clinical trial named PURPOSE 1, which showed lenacapavir achieved a 100% efficacy rate in a clinical trial targeting young women and had the convenience of being administered once every six months. The study was conducted in South Africa and Uganda and involved 5,338 cisgender women and adolescent girls aged 16 to 25. It demonstrated perfect preventive effectiveness in the 2,134 cisgender women participants, with not a single case of HIV infection occurring. In comparison, there were 16 infection cases out of 1,068 women in the Truvada group and 39 infection cases out of 2,136 women in the Descovy group.
This groundbreaking achievement not only triggered a reaction in the stock market, leading to a significant rise in Gilead's stock price, but it also impacted other HIV drug markets, as evidenced by the decline in Aidea Pharmaceutical's share price. Gilead anticipates revealing data from its Phase III trials targeting men who have sex with men by the end of 2024 or early 2025. Should the results be positive, Lenacapavir could potentially be approved for new indications by the end of 2025, with projected global peak sales ranging from $3 to $4 billion, indicating its potential as a significant new option in the HIV prevention and treatment arena.
An exhaustive analysis of Lenacapavir can provide a valuable resource for pharmaceutical companies, medical professionals, investors, and policymakers, thereby accelerating the drug development process, optimizing market strategies, enhancing intellectual property protection, improving patient care, boosting investment returns, expanding collaboration opportunities, and supporting the development and implementation of global public health strategies. This comprehensive intelligence support is crucial for driving innovation in the pharmaceutical industry and improving public health levels.
The Synapse database reveals the research and development journey of Lenacapavir
The Synapse database is a powerful information platform, offering in-depth insights and real-time updates for professionals in the pharmaceutical industry. Users can access information on drug development dynamics, clinical trial results, patent information, market potential assessment, and competitive intelligence, thereby making more informed decisions in drug development, investment planning, market strategy formulation, and clinical practice. This one-stop information service not only accelerates the discovery and commercialization of new drugs but also fosters innovation and development within the entire pharmaceutical sector.
Let us take Lenacapavir as an example to explore the type of information on new drug development that can be obtained from the Synapse database. Firstly, log in to the Synapse database. Upon reaching the homepage, enter "Lenacapavir" in the search box.
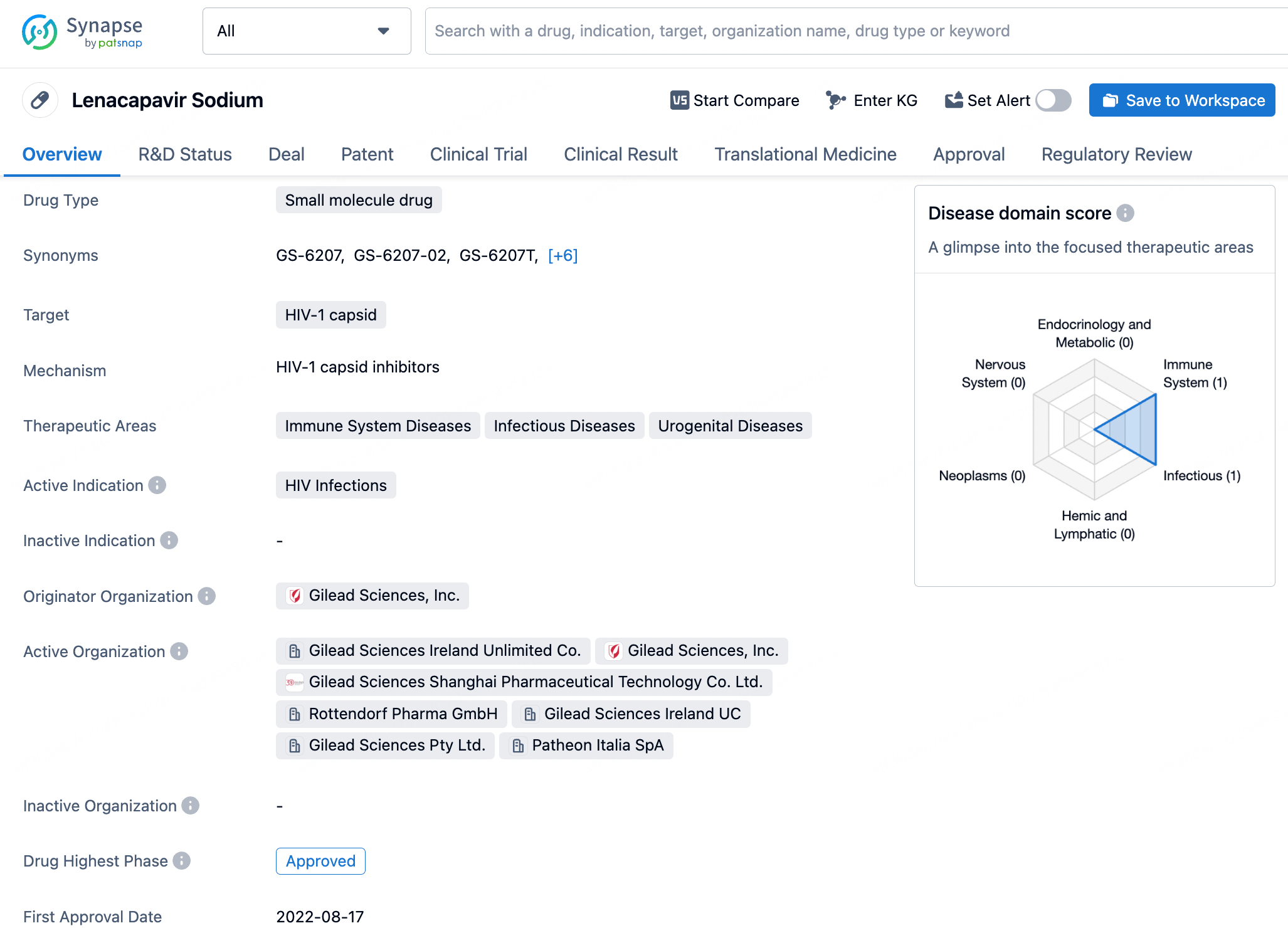
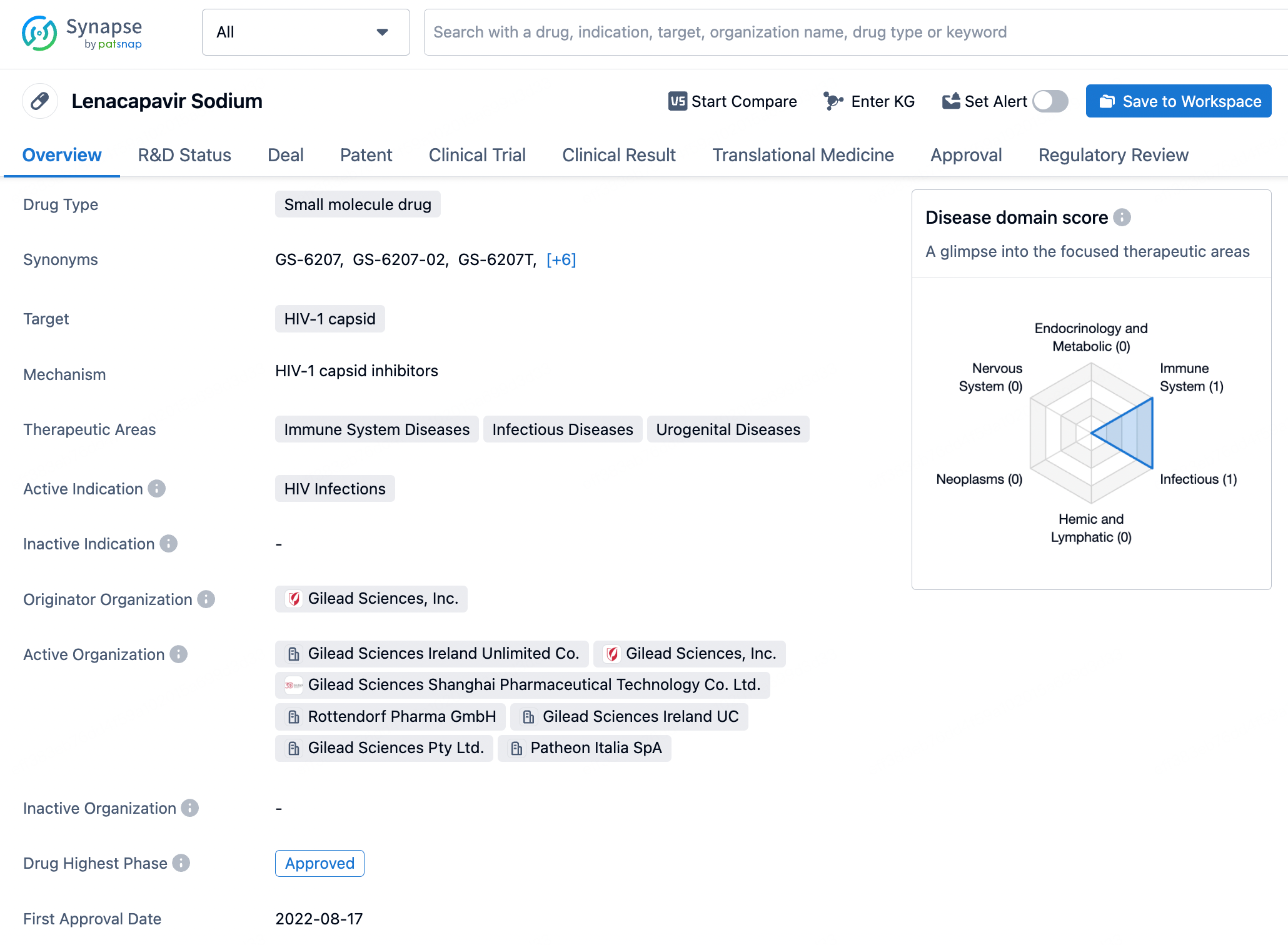
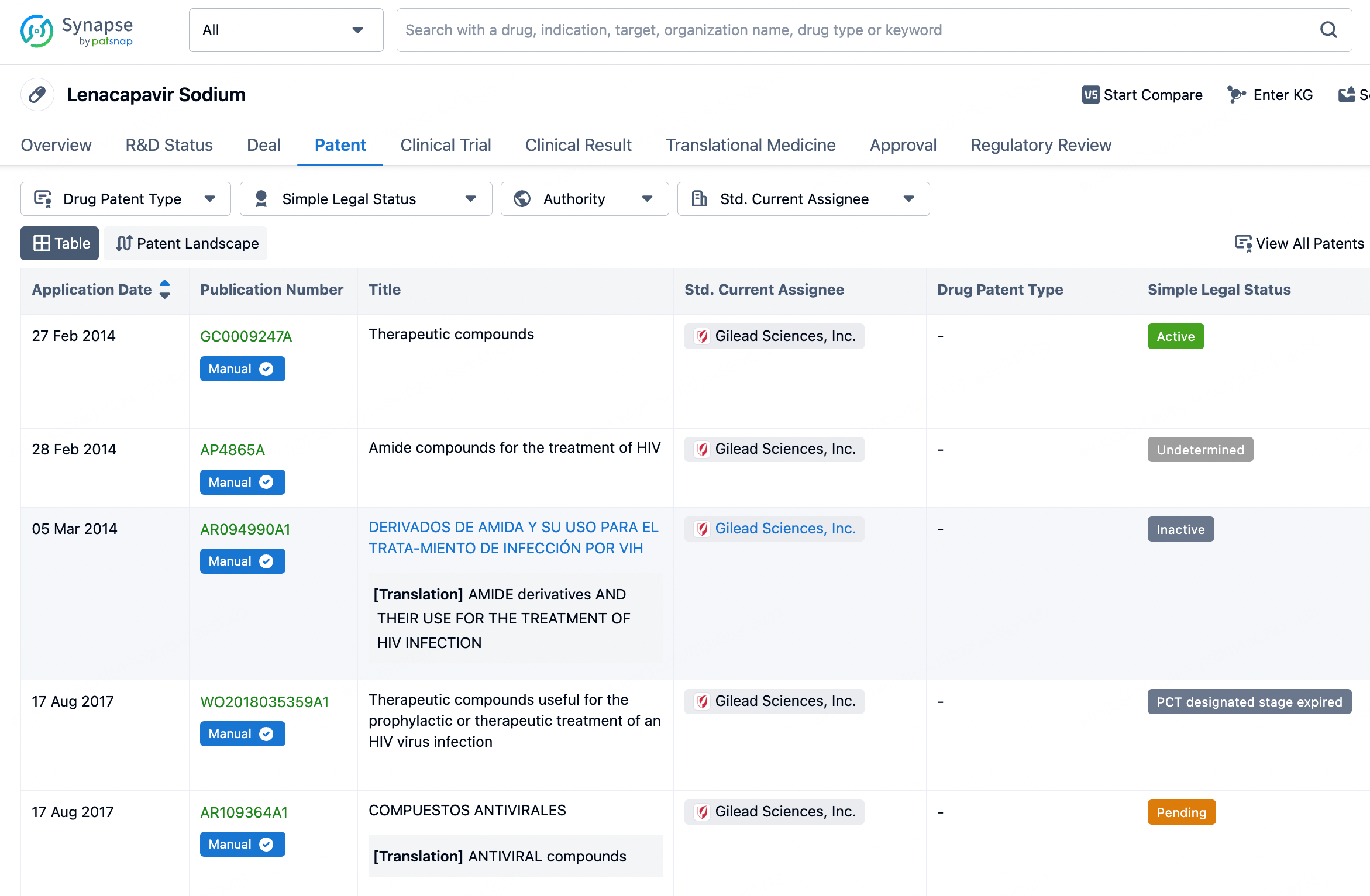
To view the details of Lenacapavir, click on it to be redirected to the drug details page, which displays basic information such as its mechanism of action, indications, and approval status. The page also organizes and presents information regarding drug transactions, core patents, clinical trial outcomes, and translational medicine.
The core patent for Lenacapavir was filed by Gilead Sciences on February 27, 2014, with the publication number GC0009247A. Since its filing date, this patent has provided a solid foundation for intellectual property protection for Lenacapavir. The expiration date of the patent is set for February 27, 2034, meaning that for the next decade or so, Gilead will hold exclusive rights to this compound, ensuring its competitive edge in the HIV treatment market. The patent protection for Lenacapavir extends beyond the United States to include multiple countries and regions globally, including China. The initial disclosure of Lenacapavir's patent revealed its use in treating HIV, offering new therapeutic options for HIV patients, particularly those who require long-term management of their viral load. As clinical trials progress and potential approvals are granted, Lenacapavir is expected to become a significant component of the HIV treatment landscape, contributing to the global efforts in combating HIV/AIDS.
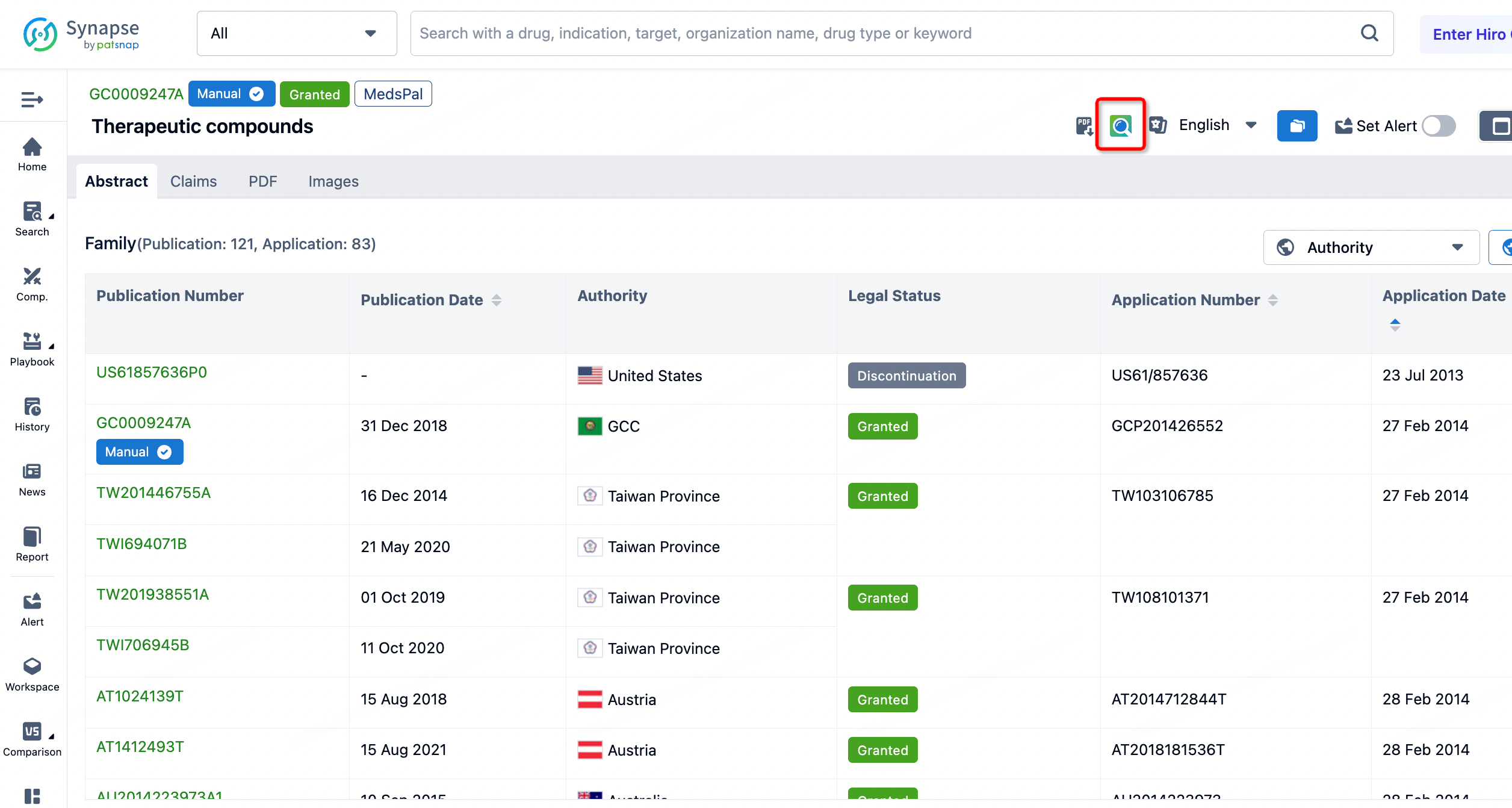
More detailed patent information can be accessed by navigating to the Patsnap database.
Once inside the Patsnap database, clicking on a specific patent publication number allows users to retrieve the patent abstract, specification, patent value, and legal information, among others. Related information includes citation data, family patents, similar patents, and related literature.
The patent value assessment tool provides users with a comprehensive evaluation of the commercial value of drugs like Lenacapavir. This tool conducts an in-depth quantitative analysis of the economic value and market prospects of the drug by considering key factors such as technical influence, legal status, market potential, citation records, and the background of the patent holder.
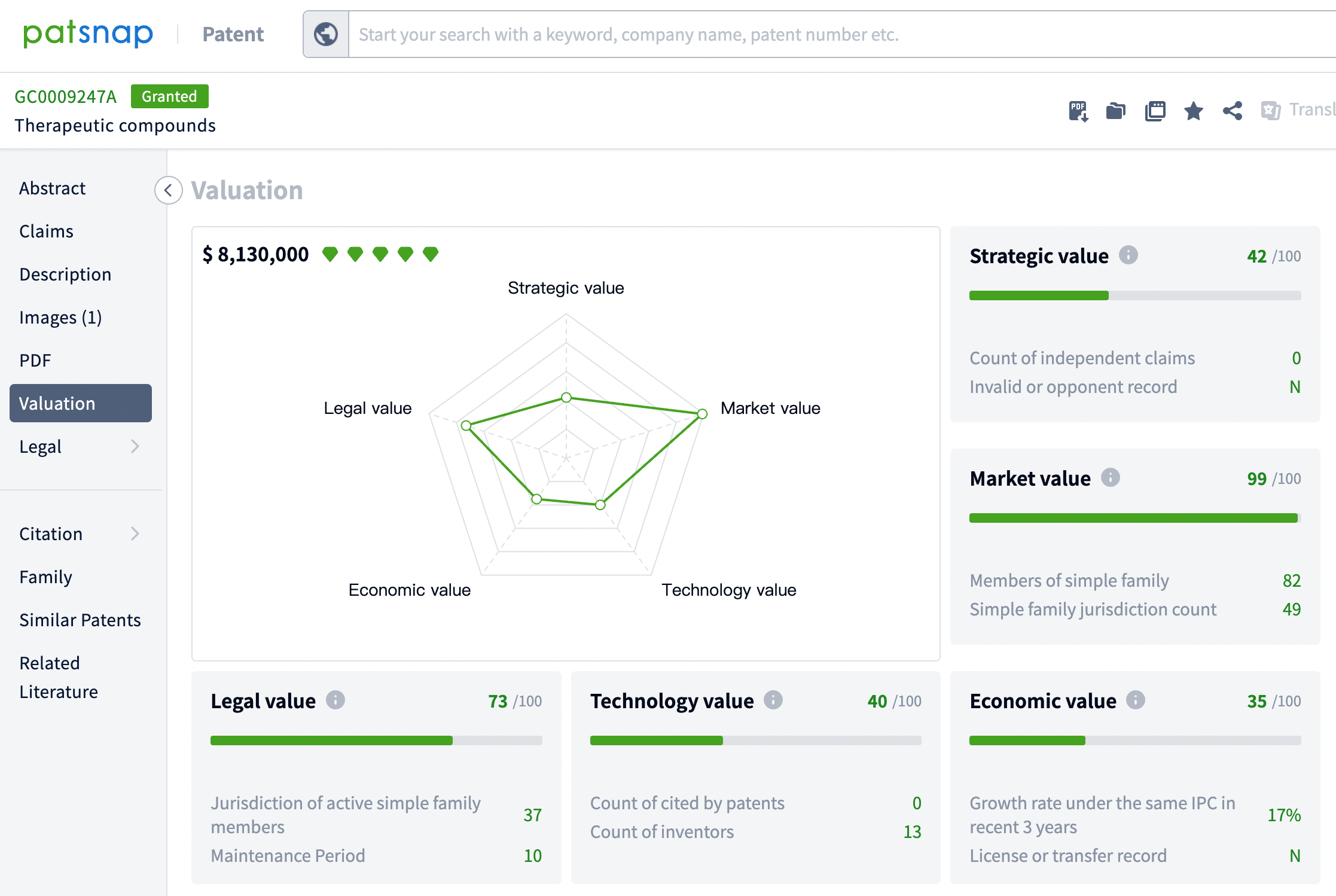
Additionally, through the search function for similar patents, 58 patents that are similar or related were discovered, of which 20 were filed by other companies. This information is crucial for assessing the originality of the patent, monitoring potential patent disputes, and formulating corresponding intellectual property strategies. These data also assist companies in technology monitoring, avoiding infringement risks, and may inspire new collaborations or R&D directions, thereby promoting innovation and development in the pharmaceutical field.
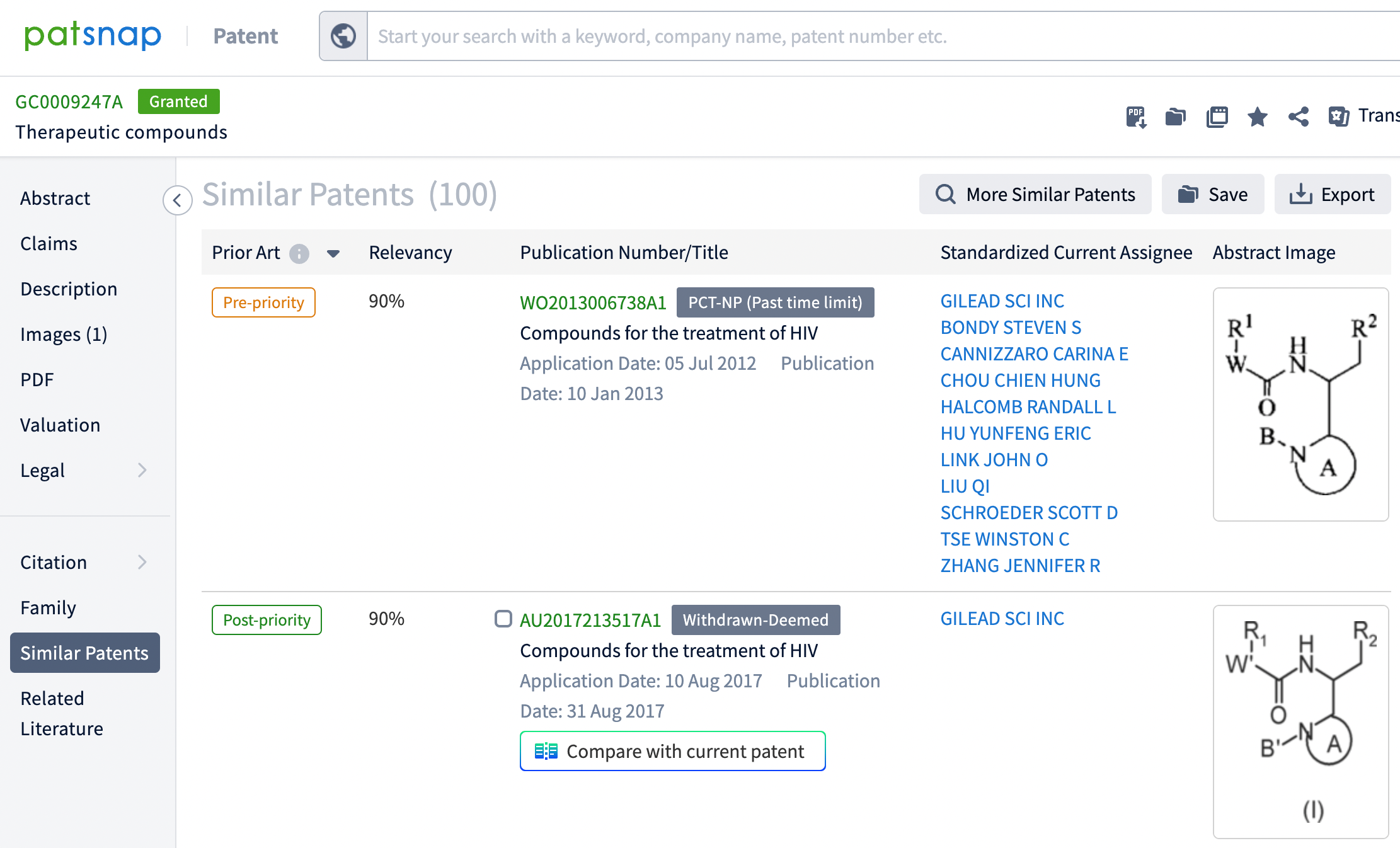
The Synapse database provides users with a comprehensive window into the development of innovative drugs. Through the Synapse database, users can track the entire process of a new drug from early research to clinical trials, and then to post-market applications. The database offers patent information, market analysis, and relevant literature, which not only reveal the commercial value and potential market prospects of the drugs but also demonstrate their profound impact in scientific and clinical research. This not only aids in optimizing R&D strategies and assessing market opportunities but also guides the effective protection of intellectual property, offering robust support for drug development and commercialization.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!