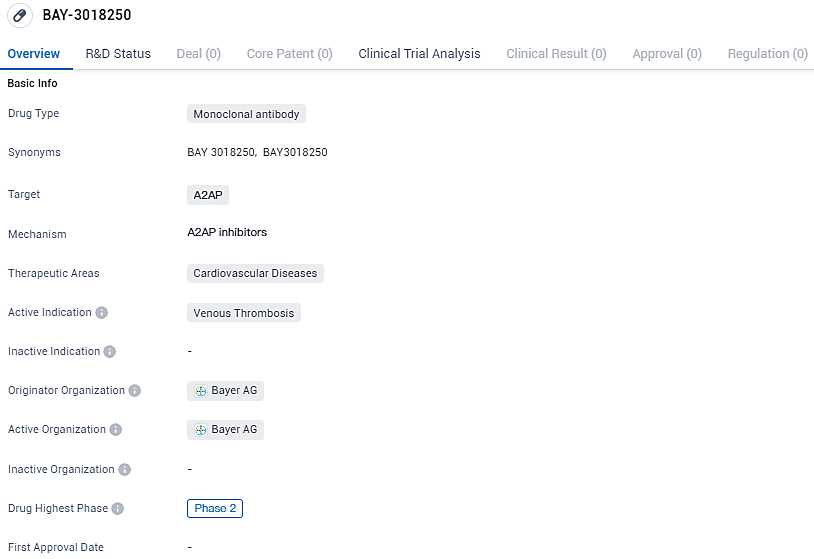
Bayer Initiates Phase II Trial of New Anti-alpha2 Antiplasmin Drug for Deep Vein Thrombosis
Bayer has initiated a Phase II clinical study to evaluate BAY3018250, a pioneering anti-alpha2 antiplasmin (anti-α2ap) antibody, in individuals diagnosed with deep vein thrombosis. This research could yield data supporting the therapeutic promise of the anti-α2ap antibody for conditions that are critically important to medical science.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"It's with great enthusiasm that we move forward with our pioneering work on the anti-α2ap antibody for the next phase of trials in individuals affected by deep vein thrombosis," announced Christian Rommel, part of the Executive Committee for Bayer AG's Pharmaceuticals Division and the chief of Research and Development.
He continued, "The upcoming trials are set to shed light on the anti-α2ap antibody's effectiveness as a thrombolytic agent for treating patients facing critical health conditions. Our strategy is to precisely manipulate plasmin activity by inhibiting alpha2 antiplasmin, aiming for selective dissolution of blood clots."
Upon the completion of the initial human trials, the aims of the blinded, placebo-controlled Phase II trial across multiple centers are to meticulously evaluate the therapeutic effect and safety profile of BAY3018250 in individuals who exhibit symptoms of proximal deep vein thrombosis. The focus of BAY3018250 is the targeted inhibition of alpha2 antiplasmin, a key regulator of plasmin's activity which plays a crucial role in maintaining clotting equilibrium within the bloodstream.
As a frontrunner in the field of cardiology, Bayer is committed to pushing the boundaries of medicinal advancements in cardiovascular diseases that currently lack sufficient treatment options. The company's strategy hinges on transforming its portfolio to harness precision in cardiology, alleviate the burden of cardiovascular diseases, and propel sustained market growth.
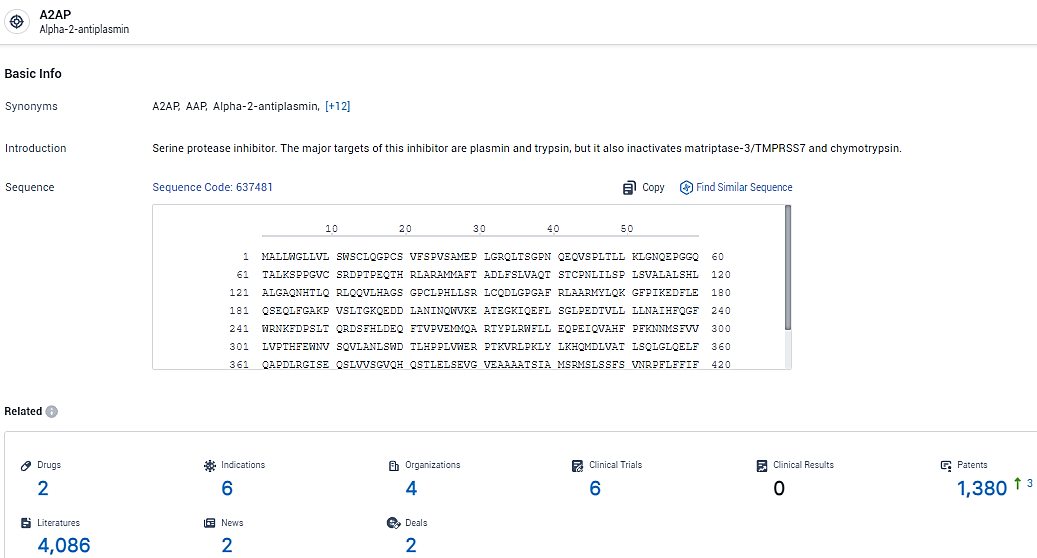
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 23, 2024, there are 2 investigational drugs for the A2AP target, including 6 indications, 4 R&D institutions involved, with related clinical trials reaching 6, and as many as 1380 patents.
BAY-3018250 targets A2AP and is intended for the treatment of venous thrombosis. It is currently in Phase 2 of clinical development, and if successful, it has the potential to address an unmet medical need in the field of cardiovascular diseases.