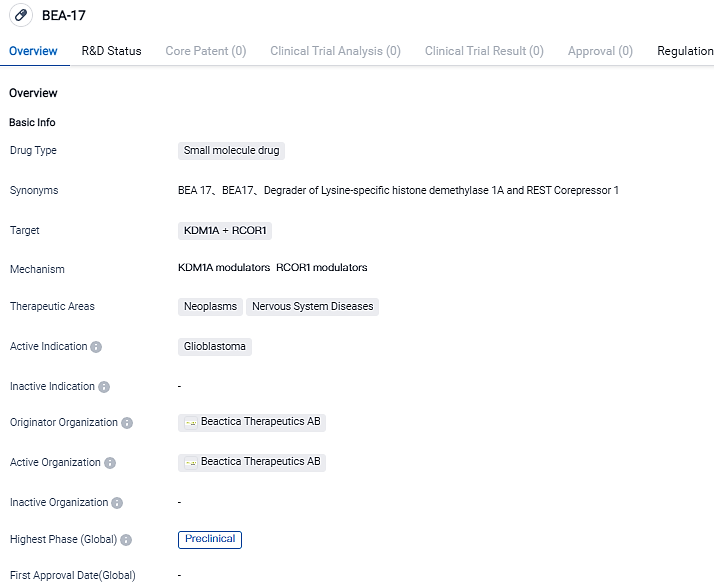
Beactica Therapeutics announces their choice of BEA-17 for the preclinical candidate status
Swedish precision oncology firm, Beactica Therapeutics AB, has today unveiled BEA-17 as their chosen preclinical candidate within the LSD1 initiative, striving to discover novel therapies to treat aggressive brain malignancies and other critical types of cancers.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
BEA-17, a pioneering small molecule, has demonstrated the potential to enhance immune-modifying therapies in preclinical models of various types of cancer. FDA has bestowed Orphan Drug Designation on BEA-17 for the treatment of glioblastoma - the most aggressive type of brain cancer.
"We are delighted to declare BEA-17 as a preclinical candidate. This significant step forward is a huge triumph, considering that many programmes do not progress past the preclinical phase." stated Dr Per Källblad, CEO of Beactica Therapeutics. "Choosing this molecule underlines our goal to expedite the testing of this innovative approach in humans."
The small molecule BEA-17 serves to degrade lysine demethylase 1. In appropriate animal models of cancer, the compound has shown significant potential to boost immune-modulating therapies. This includes anti-PD1 checkpoint inhibitors in colon cancer and the standard treatment in glioblastoma. Blood-brain-barrier penetration and oral availability are favourable in the studies of BEA-17.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
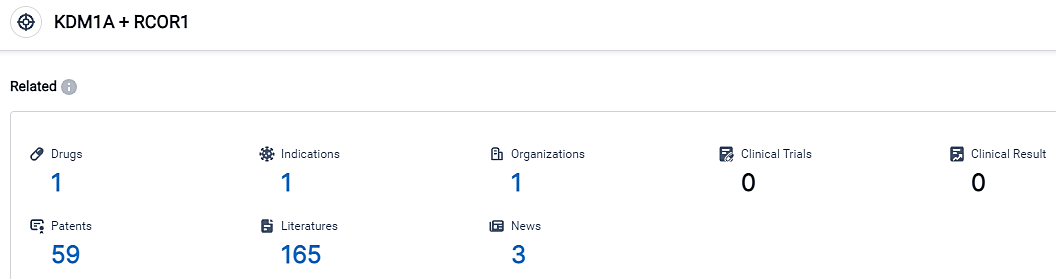 According to the data provided by the Synapse Database, As of September 6, 2023, there are 1 investigational drugs for the KDM1A and RCOR1 target, including 1 applicable indications, 1 R&D institutions involved, and as many as 59 patents.
According to the data provided by the Synapse Database, As of September 6, 2023, there are 1 investigational drugs for the KDM1A and RCOR1 target, including 1 applicable indications, 1 R&D institutions involved, and as many as 59 patents.
BEA-17 is currently under examination and has not been sanctioned worldwide. Its effectiveness and risk factors for humans are yet to be confirmed. FDA has provided BEA-17 with an Orphan Drug Designation for the management of glioblastoma.