BeiGene reports positive regulatory developments in Europe and the U.S., following the recent reacquisition of worldwide rights to TEVIMBRA®
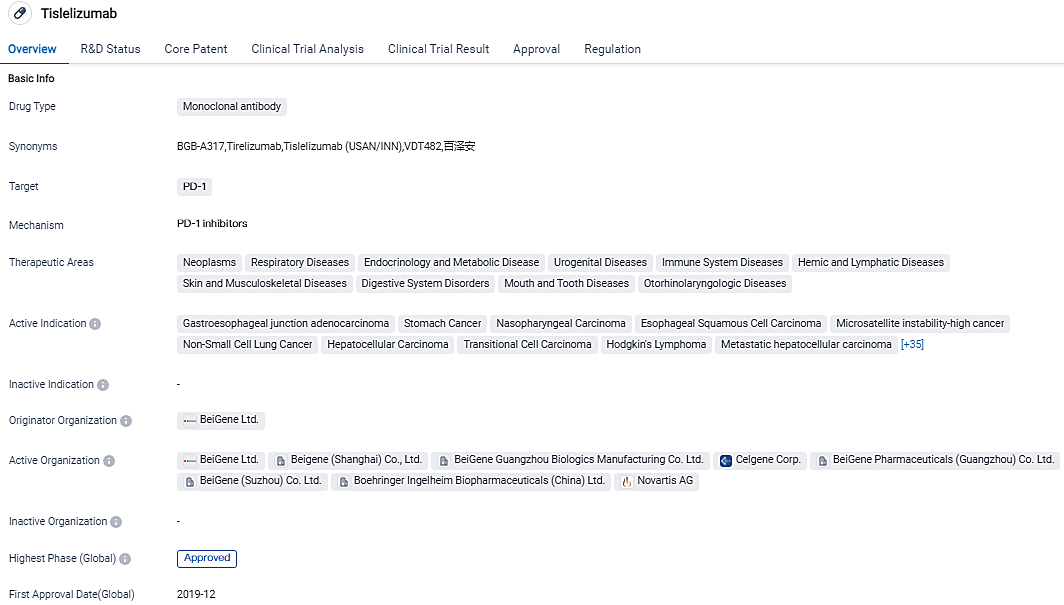
The global biotechnology firm, BeiGene, Ltd., revealed that TEVIMBRA®(tislelizumab) has received approval by the EC to be used as a single therapy for addressing unresectable, locally advanced or metastasized esophageal squamous cell carcinoma in adult patients who have previously undergone platinum-based chemotherapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The US FDA has agreed to review a Biologics License Application for tislelizumab, an innovative solution proposed as a primary line of treatment for patients suffering from inoperable, frequently recurrent, locally progressed, or metastasized ESCC. The European authorization of tislelizumab represents a key development for patients, care providers, and doctors, considering the critical necessity for fresh treatment alternatives.
Mark Lanasa, M.D., Ph.D., the Chief Medical Officer for Solid Tumors at BeiGene, expressed enthusiasm about the recent approval by the European Commission and the FDA's agreement to evaluate tislelizumab. Mark highlighted that they had recently reobtained complete global rights to this vital medication. These developments are of considerable significance for individuals with advanced or metastatic ESCC, given the proven effectiveness of tislelizumab in enhancing patient survival rates as both a monotherapy and in conjunction with chemotherapy.
BeiGene's solid tumor portfolio's crucial component is TEVIMBRA, as per Josh Neiman, Chief Commercial Officer for North America and Europe at BeiGene. In his view, total managerial control over TEVIMBRA's development and marketing will help BeiGene expedite their plans and benefit more patients internationally. Josh anticipates bringing TEVIMBRA to patients enduring advanced or metastatic ESCC, a harsh illness with limited treatment substitutes.
The FDA expects to reach a decision by the end of 2024, according to the Prescription Drug User Fee Act schedules. The FDA's assessment will involve scrutiny of the previously disclosed findings from the RATIONALE 306, a randomized, placebo-controlled, double-blind, global Phase 3 trial assessing the efficacy and safety of combining tislelizumab and chemotherapy as a primary treatment for patients with advanced or metastatic ESCC.
TEVIMBRA, a humanized IgG4 anti-PD-1 monoclonal antibody, has been specially engineered to lessen its interaction with Fc-gamma (Fcγ) receptors on macrophages, thereby enhancing the immune system's ability to detect and fight tumors. Pre-clinical studies have indicated that engagement with Fcγ receptors on macrophages can hinder the anti-tumor capacities of PD-1 antibodies by triggering antibody-mediated macrophage killing of T effector cells.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
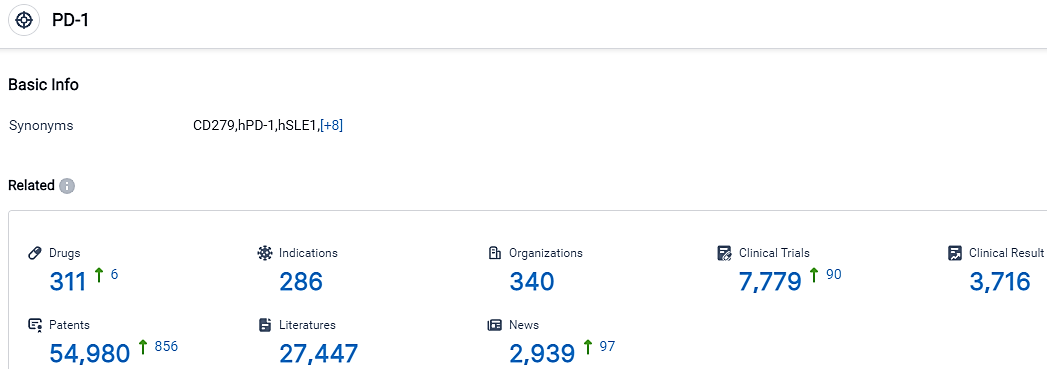
According to the data provided by the Synapse Database, As of September 20, 2023, there are 311 investigational drugs for the PD-1 target, including 286 indications,340 R&D institutions involved, with related clinical trials reaching 7779,and as many as 54980 patents.
TEVIMBRA is currently under review by the U.S. FDA and received approval by the EC for advanced or metastatic esophageal squamous cell carcinoma after prior chemotherapy. Regulatory submissions for TEVIMBRA are also under review by authorities in the U.K., Australia, China, New Zealand, Brazil, Korea, Switzerland, Israel and Indonesia. Tislelizumab is approved as a treatment in 11 indications in China and is the leading PD-1 inhibitor in the country.