ADO-Trastuzumab Emtansine Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
ADO-Trastuzumab Emtansine's R&D Progress
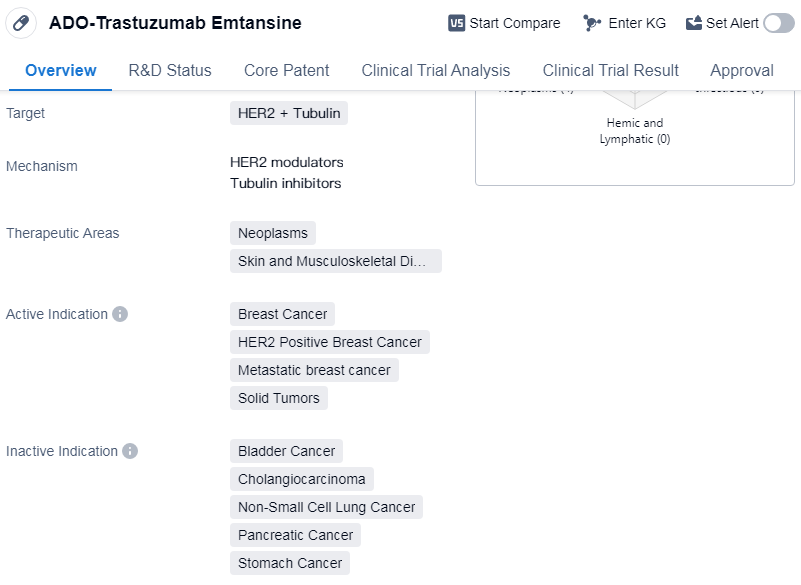
ADO-Trastuzumab Emtansine is a drug that falls under the category of monoclonal antibody and antibody drug conjugate (ADC). It is designed to target HER2 and tubulin, making it suitable for the treatment of neoplasms, skin, and musculoskeletal diseases. The drug has been primarily indicated for breast cancer, specifically HER2 positive breast cancer, metastatic breast cancer, and solid tumors.
Genentech, Inc. is the originator of ADO-Trastuzumab Emtansine. The drug has received approval globally, indicating its effectiveness and safety. The first approval for ADO-Trastuzumab Emtansine was granted in the United States in February 2013.
In terms of regulation, ADO-Trastuzumab Emtansine has undergone priority review, fast track, and breakthrough therapy designations.
ADO-Trastuzumab Emtansine represents a significant advancement in the field of biomedicine, particularly in the treatment of breast cancer. By targeting HER2 and tubulin, it offers a targeted approach to combating the disease. The drug's approval in the global market highlights its potential to benefit patients worldwide.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for ADO-Trastuzumab Emtansine: HER2 modulators and Tubulin inhibitors
From a biomedical perspective, HER2 modulators refer to drugs or substances that specifically target and modulate the human epidermal growth factor receptor 2 (HER2). HER2 is a protein that is overexpressed in certain types of cancer cells, particularly breast cancer cells. HER2 modulators can either block or activate the HER2 receptor, depending on the specific mechanism of action of the drug. By modulating HER2, these drugs aim to inhibit the growth and proliferation of cancer cells.
Tubulin inhibitors, on the other hand, are drugs that target tubulin, a protein involved in the formation of microtubules. Microtubules are essential structures for cell division, and tubulin inhibitors disrupt their formation or function. By interfering with microtubule dynamics, tubulin inhibitors prevent cancer cells from dividing and proliferating. These drugs are commonly used in the treatment of various types of cancer, including breast, lung, and ovarian cancer.
In summary, HER2 modulators are drugs that target the HER2 receptor in cancer cells, while tubulin inhibitors target tubulin and disrupt microtubule formation, both playing a crucial role in cancer treatment.
Drug Target R&D Trends for ADO-Trastuzumab Emtansine
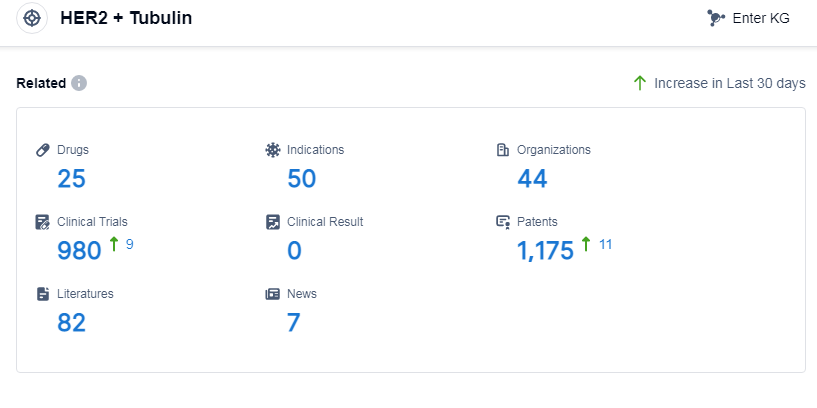
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 25 HER2 and tubulin drugs worldwide, from 44 organizations, covering 50 indications, and conducting 980 clinical trials.
The analysis of the current competitive landscape of target HER2 and tubulin reveals that RemeGen Co., Ltd., Roche Holding AG, Zydus Lifesciences Ltd., and Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. are the leading companies in terms of growth and development stages. These companies have made significant progress in R&D, with drugs in various phases. The approved indications for drugs targeting HER2 and tubulin cover a wide range of cancers, including breast cancer, gastric cancer, and lung cancer. The drug types progressing rapidly include monoclonal antibodies, antibody drug conjugates (ADC), and biosimilars. China is a key player in the development of drugs targeting HER2 and tubulin, with the highest number of drugs in different phases. The future development of target HER2 and tubulin is promising, with ongoing research and development efforts by multiple companies and countries.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, the information provided offers a factual overview of ADO-Trastuzumab Emtansine, drug type, targets, therapeutic areas, active indications, originator organization, approval status, and regulatory designations. It is important to note that this summary is based solely on the given information and does not include any subjective interpretations or fictional data.