BeiGene Secures EC Nod for Tislelizumab in Treating Non-Small Cell Lung Cancer
BeiGene, Ltd., an international firm specializing in oncology, disclosed that tislelizumab has received approval from the European Commission for use in treating non-small cell lung cancer. This authorization covers three specific uses, encompassing both initial and subsequent treatments.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Tislelizumab serves as a cornerstone in BeiGene's portfolio for solid tumors, showing promise across diverse tumor types such as NSCLC, where significant needs remain unaddressed at every stage," noted Mark Lanasa, M.D., Ph.D., Head of Solid Tumor Division at BeiGene.
Mark Lanasa further noted, "The EC's recent approval signifies a key milestone for tislelizumab, marking its second authorization within this region. It is now approved for both NSCLC and for the treatment of locally advanced or metastatic esophageal squamous cell carcinoma in the European Union. Additionally, its use as a second-line treatment for ESCC was recently cleared by the U.S. FDA, advancing our mission to deliver this innovative treatment to a broader patient base globally."
Tislelizumab is combined with carboplatin and either paclitaxel or nab-paclitaxel for the initial therapy of adult patients with squamous NSCLC, who present with locally advanced disease or non-operable tumors, or who have metastatic disease.
Additionally, the drug is used in combination with pemetrexed and platinum-based chemotherapy for treating adult patients with non-squamous NSCLC, where tumors exhibit a PD-L1 expression in ≥50% of the cells, excluding those with EGFR or ALK mutations, and who have advanced disease stages unsuitable for surgery or platinum-based chemoradiation.
Authorized under the name TIZVENI for these NSCLC usages, BeiGene intends to amalgamate these with the second-line ESCC indication into the TEVIMBRA brand. Launches in the initial EU territories are expected in late 2024. TEVIMBRA has gained approval in the U.S. and EU for treating advanced or metastatic ESCC following previous chemotherapy. It is currently under assessment by the European Medicines Agency and the U.S. FDA as a primary treatment option for patients with inoperable or recurrent, locally advanced, or metastatic ESCC, and for initial treatment of gastric or gastroesophageal junction cancers.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
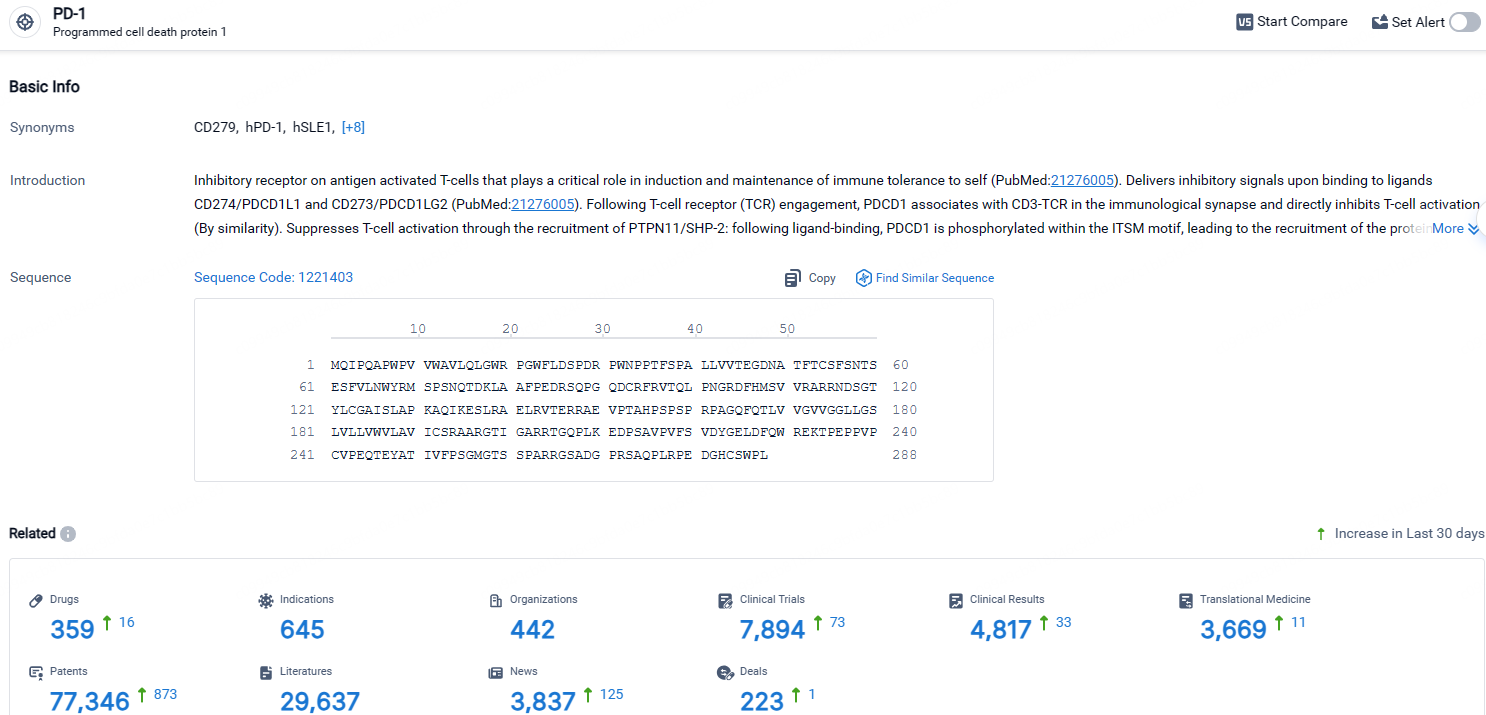 According to the data provided by the Synapse Database, As of April 25, 2024, there are 359 investigational drugs for the PD-1 targets, including 645 indications, 442 R&D institutions involved, with related clinical trials reaching 7894, and as many as 77346 patents.
According to the data provided by the Synapse Database, As of April 25, 2024, there are 359 investigational drugs for the PD-1 targets, including 645 indications, 442 R&D institutions involved, with related clinical trials reaching 7894, and as many as 77346 patents.
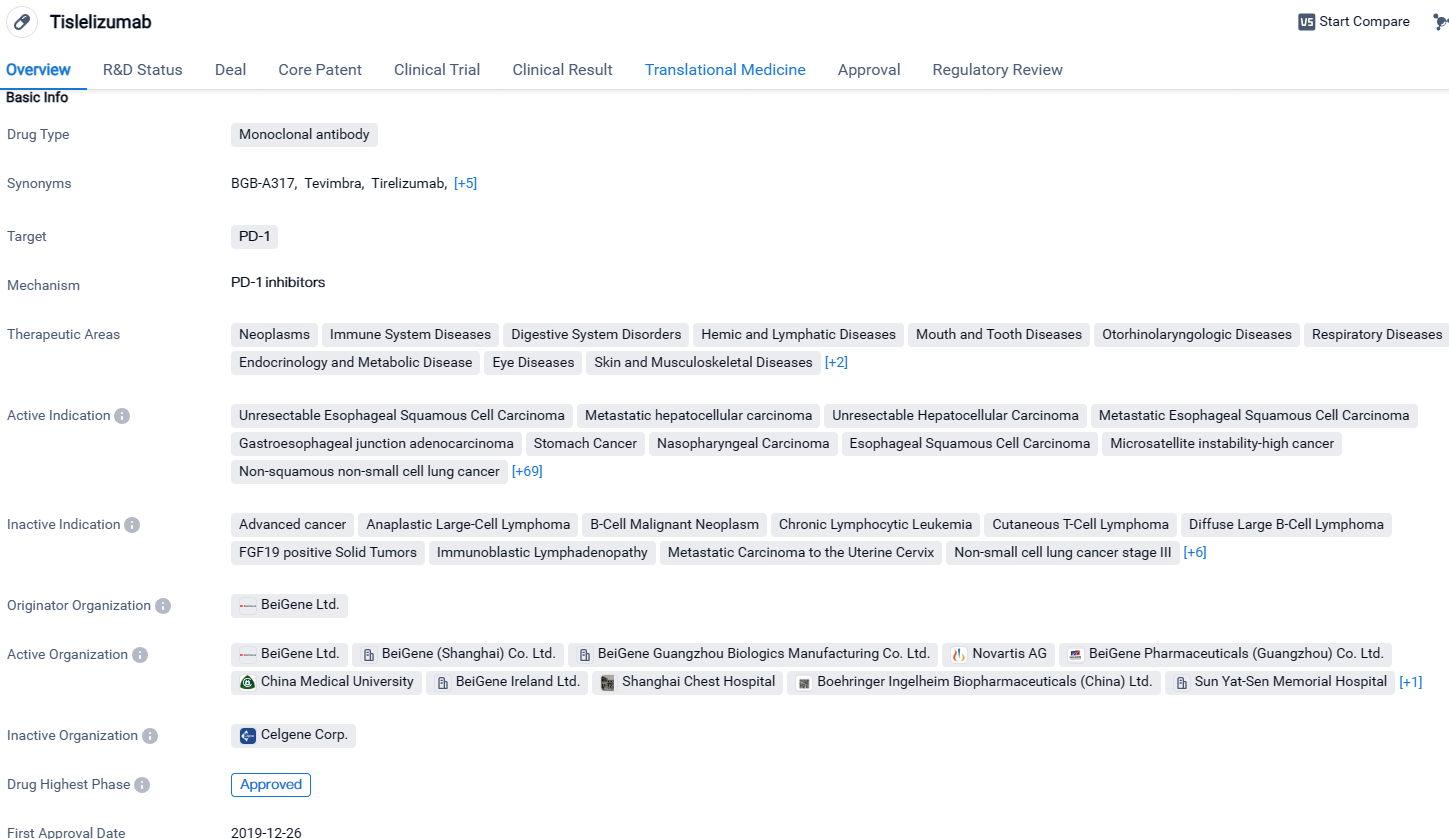
Tislelizumab is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of various types of cancer. It has shown promising results in clinical trials and has received regulatory approvals in China. The drug's wide range of therapeutic areas and indications highlight its potential in addressing multiple diseases within the field of biomedicine.