EU Sanctions Voydeya as Supplementary Treatment for PNH with Persistent Haemolytic Anaemia
Voydeya (danicopan) has received approval within the European Union to be used alongside ravulizumab or eculizumab as a treatment for adult patients suffering from paroxysmal nocturnal haemoglobinuria with ongoing haemolytic anaemia. Voydeya, a pioneering oral Factor D inhibitor, is designed to complement the existing treatments Ultomiris (ravulizumab) or Soliris (eculizumab). This medication primarily targets the 10-20% of PNH patients who continue to display substantial extravascular haemolysis despite receiving a C5 inhibitor.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The European Commission has sanctioned the use of a new medicinal product following the favorable recommendation by the Committee for Medicinal Products for Human Use. This authorization is underpinned by findings from the crucial ALPHA Phase III study. The findings during a 12-week initial assessment phase were detailed in The Lancet Haematology.
Speaking on the matter, Marc Dunoyer, the Chief Executive Officer at Alexion, remarked, "For individuals battling PNH, the introduction of Voydeya as supplementary therapy has successfully addressed key signs and symptoms of significant EVH, which includes anaemia, alongside basing treatment on Soliris or Ultomiris. We are eager to deliver this pioneering Factor D inhibitor to European patients and are committed to extending availability internationally.”
The ALPHA Phase III study probed the effectiveness and tolerability of Voydeya when used alongside Ultomiris or Soliris in PNH patients who showed significant EVH. Outcomes indicated that Voydeya achieved its primary goal of adjusting hemoglobin levels from the beginning to week 12 and triumphed in all major secondary objectives, including reducing the need for transfusions and ameliorating the Functional Assessment of Chronic Illness Therapy – Fatigue rating.
Further findings from the same trial indicated that Voydeya was generally well-received with no emergent safety worries. Throughout the study, common side effects encountered included headache, nausea, joint pain, and diarrhea.
Regulatory bodies have recognized Voydeya’s therapeutic potential: the U.S. Food and Drug Administration has awarded it a Breakthrough Therapy designation and the European Medicines Agency has endowed it with PRIority MEdicines status. Additionally, the treatment has received Orphan Drug Designation for the management of PNH in the United States, European Union, and Japan. Voydeya has obtained approval in the United States and Japan, while regulatory assessments continue in other nations.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
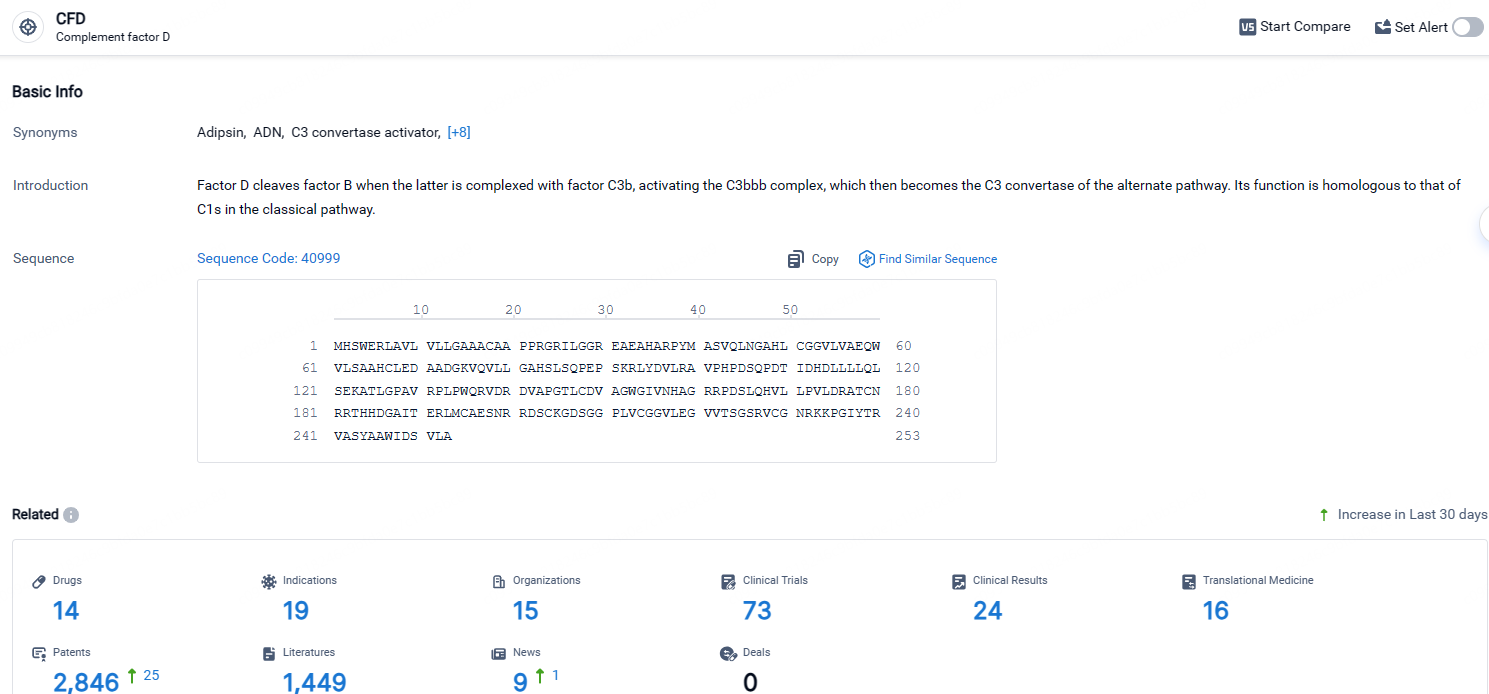
According to the data provided by the Synapse Database, As of April 24, 2024, there are 14 investigational drugs for the CFD targets, including 19 indications, 15 R&D institutions involved, with related clinical trials reaching 73, and as many as 2846 patents.
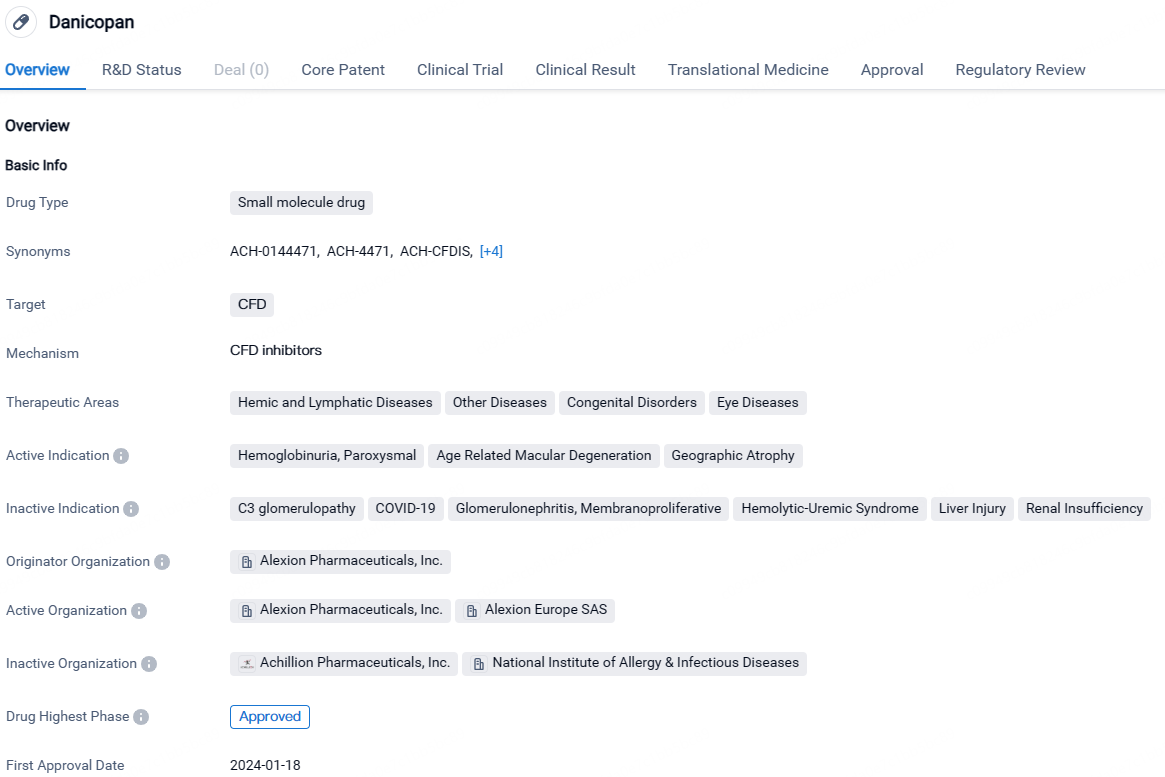
Danicopan is a small molecule drug developed by Alexion Pharmaceuticals, Inc. It targets CFD and is indicated for the treatment of hemoglobinuria, paroxysmal, age-related macular degeneration, and geographic atrophy. The drug has received approval in Japan and has undergone various regulatory processes, including pediatric investigation plan, PRIME designation, breakthrough therapy designation, and orphan drug status.