BeiGene Wins US Approval for TEVIMBRA® in Post-Chemo Esophageal Cancer
BeiGene, Ltd., an international company specializing in cancer treatment solutions, has made an announcement regarding the sanction by the U.S. Food and Drug Administration for their drug, TEVIMBRA® (tislelizumab-jsgr). This medication is now authorized for solo use for adult individuals who are dealing with inoperable or metastatic esophageal squamous cell carcinoma and have previously undergone systemic chemotherapy, with the exception of therapies involving PD-(L)1 inhibitors. Patients in the United States can expect to access TEVIMBRA starting in the latter part of the year 2024.
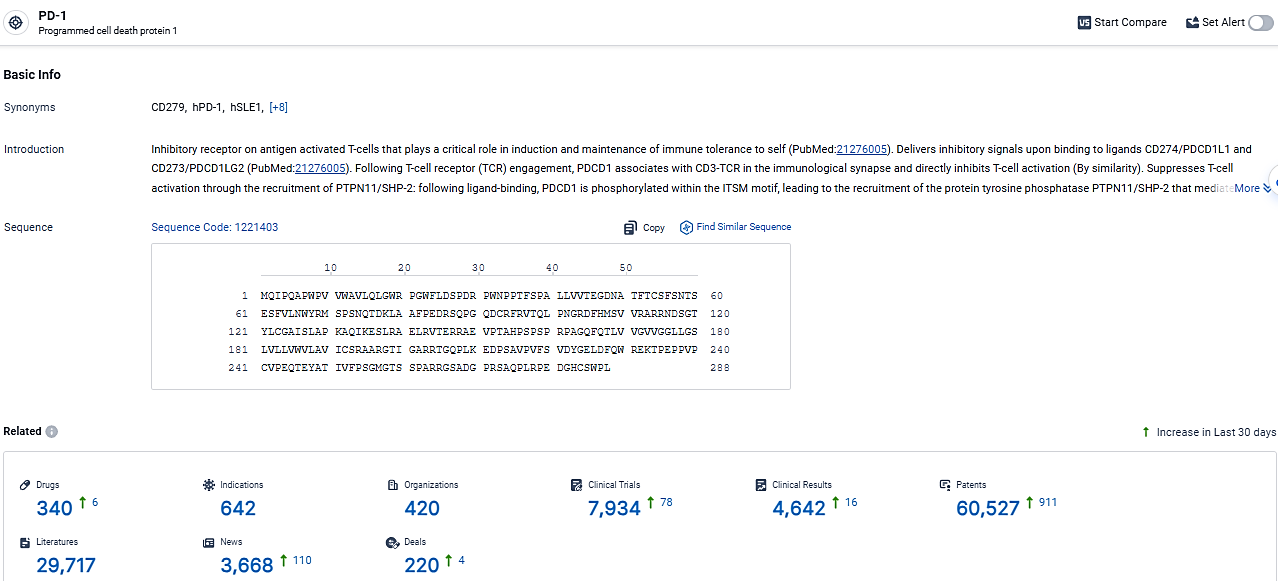
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Mark Lanasa, M.D., Ph.D., the Chief Medical Officer for Solid Tumors at BeiGene, announced the FDA's endorsement of TEVIMBRA for ESCC subjects who had undergone prior chemotherapy. He emphasized the ongoing examination of our BLA for the first-line treatment of ESCC patients as a pivotal advancement in our effort to provide this innovative therapy to individuals worldwide.
Dr. Lanasa added that TEVIMBRA, originating from BeiGene's immuno-oncology pipeline and being the second drug authorized in the U.S., forms an essential component of our comprehensive development strategy for solid tumors. We are currently spearheading over 17 pivotal trials across more than 30 countries, affirming our global reach in clinical research.
The endorsement of TEVIMBRA stemmed from the results of the RATIONALE 302 study. In this study, TEVIMBRA demonstrated a significant extension in survival, which was both statistically and clinically meaningful when contrasted with previous chemotherapy treatments, fulfilling the primary objective among all participants who were treated.
The European Commission sanctioned tislelizumab in 2023 for patients with advanced or metastatic ESCC who have received chemotherapy earlier. Furthermore, in February 2024, the Committee for Medicinal Products for Human Use at the European Medicines Agency issued a favorable appraisal, recommending tislelizumab for three types of non-small cell lung cancer.
Moreover, the FDA is examining biologic licensing submissions for tislelizumab as a potential first-line intervention for individuals battling with unresectable, recurrent, locally advanced, or metastatic ESCC, as well as for those with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. The deadlines for these assessments are slated for July and December of 2024 respectively."
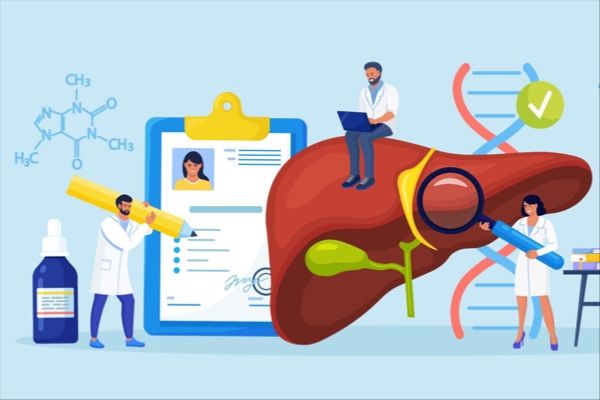
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 17, 2024, there are 340 investigational drugs for the PD1 target, including 642 indications, 420 R&D institutions involved, with related clinical trials reaching 7934, and as many as 60527 patents.
Tislelizumab is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of various types of cancer and other diseases. Its approval in China and globally highlights its potential as a therapeutic option for patients in need.