Bio-Thera Solutions announced positive results from its Phase 3 trial of BAT2206, a potential Stelara® biosimilar
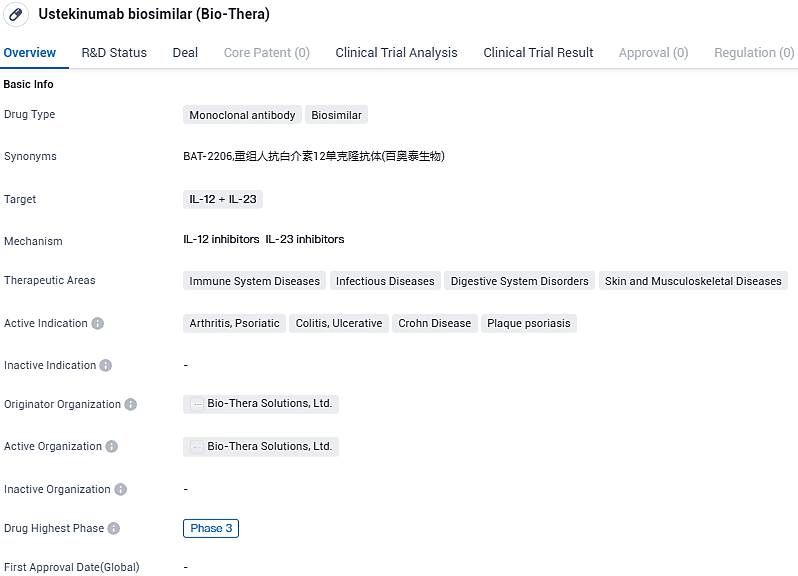
Bio-Thera Solutions, Ltd., an enterprise focused on biopharmaceutical production with a portfolio that includes both biosimilar candidates and novel compounds, has reported findings from its Phase 3 clinical trial for BAT2206, a candidate biosimilar to Stelara® (ustekinumab).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This investigation's key measure was the reduction in the Psoriasis Area and Severity Index score at the 12-week mark from the starting point, confirming that BAT2206 closely matches Stelara® for individuals suffering from moderate to significant plaque psoriasis.
Stelara® holds approval in the United States for use in individuals age 6 and above with active psoriatic arthritis, for those within the same age range grappling with moderate to intense plaque psoriasis who might benefit from phototherapy or systemic treatment, for adults with moderately to severely active Crohn's disease, and also for adults dealing with moderately to severely active ulcerative colitis.
The clinical evaluation for BAT2206 was an internationally coordinated, multi-site, randomization-based, blinded for double-observation, and dual-arm Phase 3 investigation, tasked with assessing the comparability in terms of efficacy, safety profile, immunogenic response, and pharmacokinetics between BAT2206 and Stelara®, involving 556 participants with moderate to critical plaque psoriasis.
Bio-Thera Solutions' CEO, Shengfeng Li, Ph.D., expressed, "It brings us great satisfaction to disclose our fourth proposed biosimilar's encouraging results from a Phase 3 trial. These findings indicate that our suggested biosimilar of ustekinumab can be both a dependable and effectual option for treatment.'' Dr. Li further stated, “Bio-Thera is vested in enhancing the availability of pioneering treatments by forging biosimilars of superior quality."
Expressing his delight in aiding the triumphant worldwide Phase 3 investigation of BAT2206, professor Min Zheng, a pivotal figure in that study, noted, "BAT2206 unveils the prospect of a cost-effective therapeutic alternative for patients around the world who are in urgent need of it. My congratulations go to the team at Bio-Thera Solutions for their achievement."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
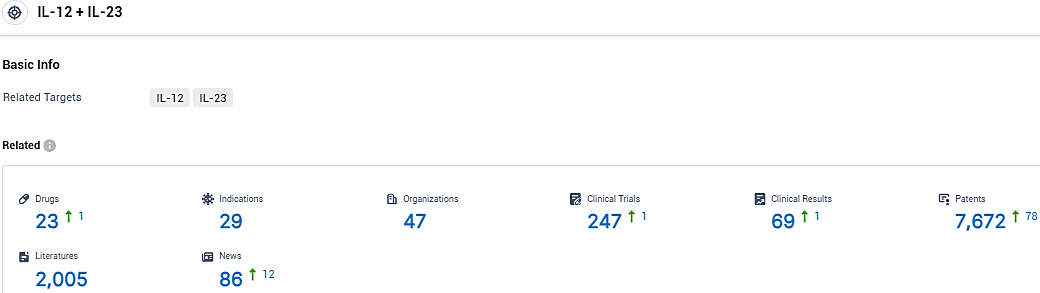
According to the data provided by the Synapse Database, As of December 7, 2023, there are 23 investigational drugs for the IL-12 and IL-23 target, including 29 indications, 47 R&D institutions involved, with related clinical trials reaching 247, and as many as 7672 patents.
BAT2206 is a proposed biosimilar to Janssen's Stelara® which is a human monoclonal antibody that inhibits the bioactivity of human IL-12 and IL-23 by preventing shared p40 from binding to the IL-12Rβ1 receptor protein expressed on the surface of immune cells. Bio-Thera entered into a commercialization and license agreement with Hikma for BAT2206 in August 2021. Developed by Bio-Thera, BAT2206 will be commercialized by Hikma in the United States of America.