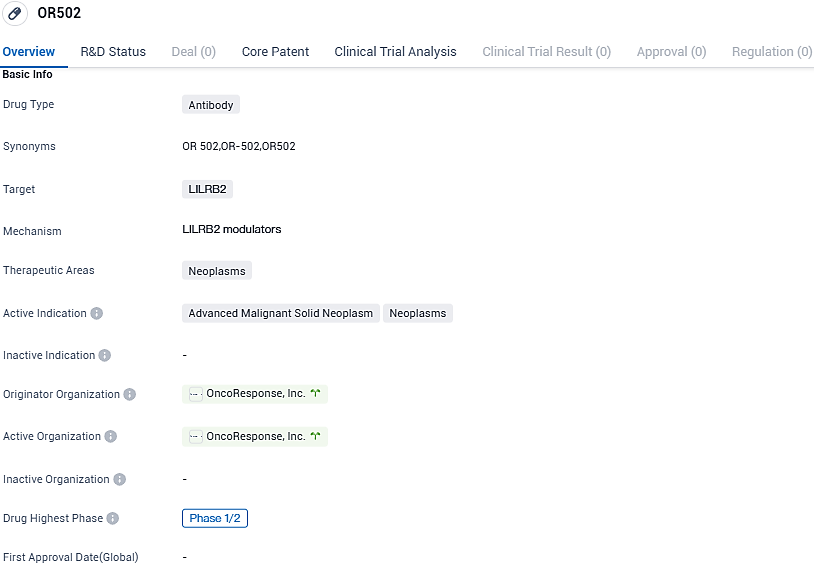
OncoResponse Begins Early Stage Clinical Study for OR502, an Anti-LILRB2 Therapy, in Patients with Progressive Malignancies
OncoResponse, an innovator in the clinical-stage biotechnology sector focusing on harnessing the power of immunotherapy treatments developed from the immune responses of elite cancer survivors, has proclaimed the administration of the initial dose to a participant in the combined Phase 1/2 study of OR502. This pioneering therapy is composed of a humanized antibody that targets the anti-leukocyte immunoglobulin like receptor B2 (LILRB2), aiming to rejuvenate both innate and adaptive immune mechanisms that have been compromised by LILRB2-induced immunosuppression.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The objective of this initial clinical trial, a combined Phase 1/2 investigation, is to assess the safety profile, tolerability levels, and early efficacy in tumor suppression by OR502, both as a single agent and when used synergistically with anti-PD-1 therapy in participants with progressive solid tumors.
"At OncoResponse, commencing this investigative trial in individuals afflicted with cancer underscores our unwavering dedication to forwarding the advancement of therapeutic approaches that hold the promise of enhancing patient conditions during oncologic treatments," commented Clifford Stocks, holding the position of CEO at OncoResponse.
"Engaging the LILRB2 receptor, OR502 disrupts the typical immune-inhibitory mechanisms within the oncological environment, providing a novel therapeutic avenue for patients who face challenges with the effectiveness of CPI treatments," stated Kamal Puri, PhD, who occupies the role of Chief Scientific Officer at OncoResponse.
Further elaborating, Kamal Puri remarked, "The dedicated team at OncoResponse has invested considerable time in devising therapeutic interventions aimed at neutralizing or altering the behavior of tumor-associated macrophages (TAMs) to induce tumor-targeting responses. The potential application of OR502 within the realm of LILRB2-targeted interventions is particularly promising, with preclinical trials demonstrating its superior performance when compared to existing anti-LILRB2 antibodies with clinical validation."
In a number of malignant states, heightened levels of LILRB2 have been found to negatively correlate with patient longevity. By focusing on LILRB2, our human-derived monoclonal antibody known as OR502 impedes the suppressive actions of TAMs while simultaneously promoting the cytotoxic behavior of T cells, thus overcoming the resistance commonly observed with CPI therapeutic regimens.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
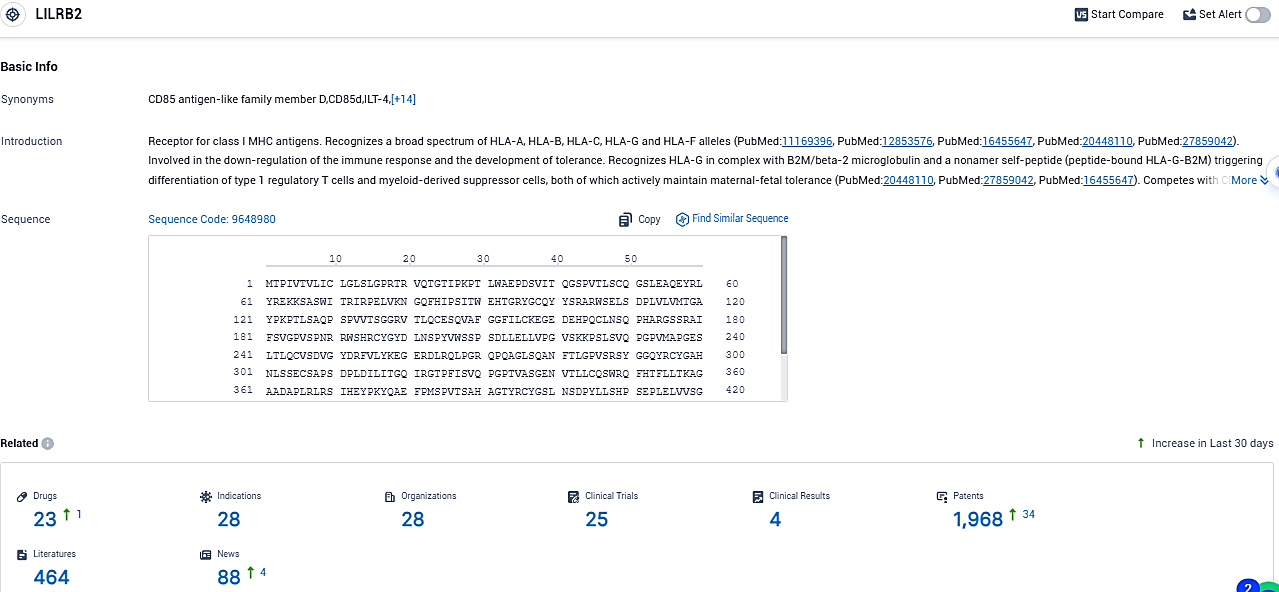
According to the data provided by the Synapse Database, As of December 7, 2023, there are 23 investigational drugs for the LILRB2 target, including 28 indications, 28 R&D institutions involved, with related clinical trials reaching 25, and as many as 1968 patents.
The development of OR502 in Phase 1/2 indicates that it is still in the early stages of clinical testing. Further research and trials will be needed to determine its safety and efficacy in a larger patient population. If successful, OR502 could potentially offer a new treatment option for patients with advanced malignant solid neoplasms and other neoplastic diseases. However, it is important to note that without additional information or data, it is difficult to make any definitive conclusions about the drug's potential impact.