BioAtla to Present Promising Phase 1 Evalstotug Trial Results at 2024 ASCO Conference
BioAtla, Inc. revealed additional findings in their upcoming presentation titled, “Phase 1 study of evalstotug (BA3071), an anti-CTLA-4 Conditionally Active Biologic, in combination with nivolumab in advanced solid tumors,” showcasing confirmed responses and a potentially unique tolerability profile of their innovative, conditionally active anti-CTLA-4 agent, evalstotug, when combined with anti-PD-1 therapy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Based on the emerging clinical profile, we are confident that evalstotug has the potential to become a best-in-class CTLA-4 antibody and may be utilized as frequently as a PD-1 inhibitor,” stated Jay M. Short, Ph.D., Chairman, CEO, and co-founder of BioAtla, Inc. “Remarkably, we have witnessed prolonged progression-free survival (PFS) exceeding 10 months and confirmed responses at higher doses of evalstotug, indicating that increased CTLA-4 blockade exposure in conjunction with PD-1 inhibition provides clinical advantages. We are currently enrolling patients in the Phase 2 first-line melanoma and mutant non-small cell lung cancer (NSCLC) combination cohorts at a 700 mg flat dose, and we anticipate moving to a 1 gram flat dose in June, following the clearance of the dose-limiting toxicity (DLT) observation period. We remain on schedule for data readouts for both monotherapy and combination treatments later this year.” Jay M. Short added.
Evalstotug is a CAB anti-CTLA-4 antibody developed as an immuno-oncology agent aimed at achieving efficacy comparable to approved anti-CTLA-4 antibodies but with reduced toxicities due to CAB’s tumor microenvironment-restricted activity. This characteristic may permit safer combination therapies of anti-CTLA-4 and anti-PD-1 checkpoint inhibitors, potentially expanding the patient population that can tolerate combination therapies and improving overall efficacy. Similar to our other CAB candidates, this Phase 2 clinical asset is designed to be conditionally and reversibly active within the tumor microenvironment. Evalstotug is being developed as a potential treatment for various solid tumors that are known to respond well to CTLA-4 treatment in combination with a PD-1 blocking agent."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
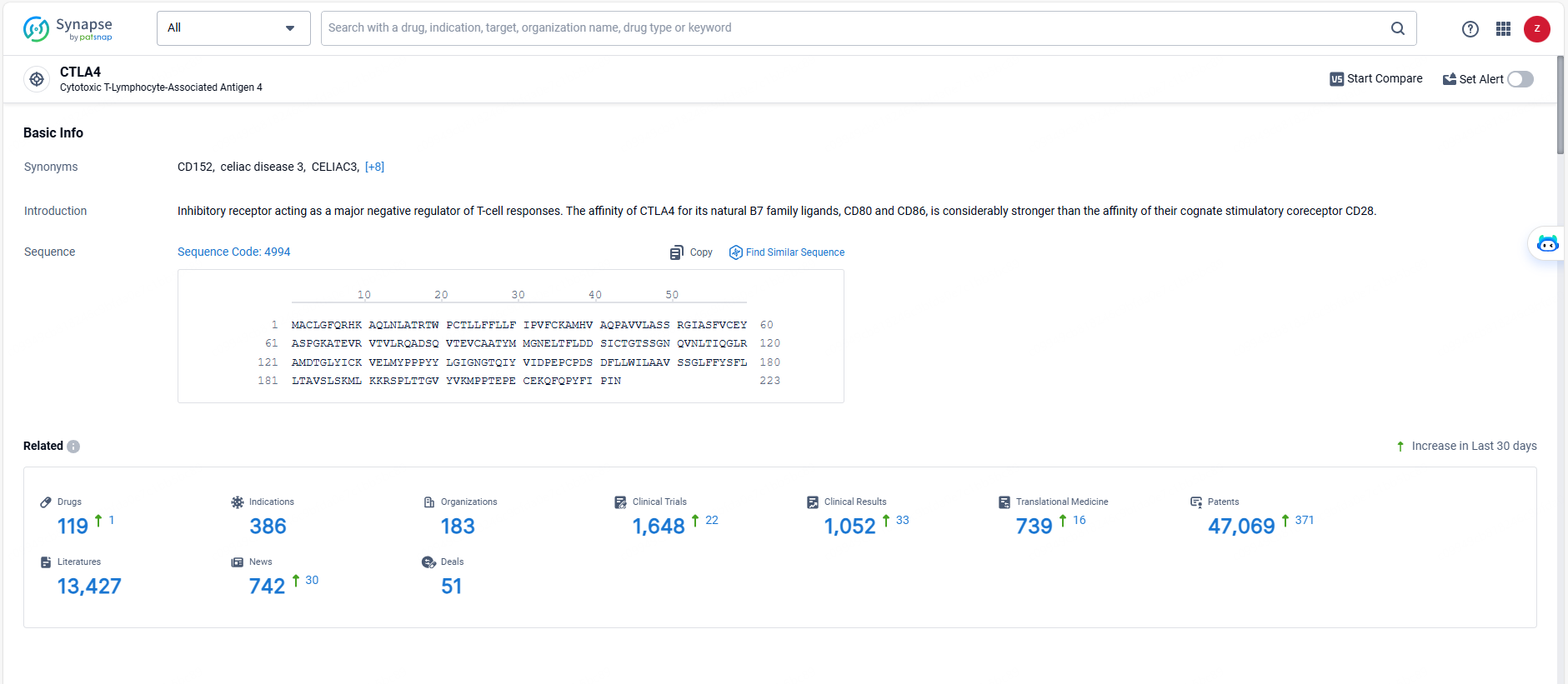
According to the data provided by the Synapse Database, As of May 29, 2024, there are 119 investigational drugs for the CTLA4 target, including 386 indications, 183 R&D institutions involved, with related clinical trials reaching 1648, and as many as 47069 patents.
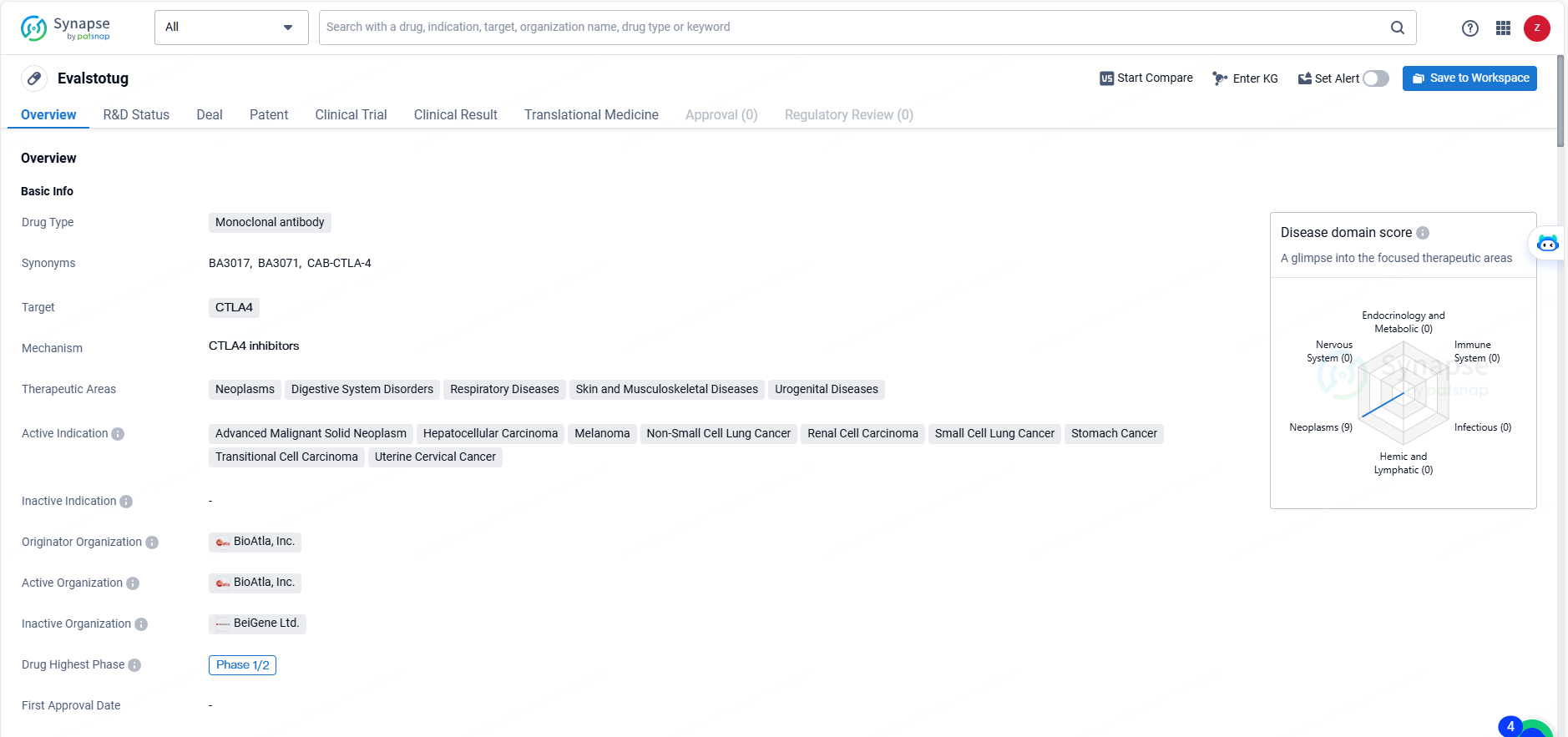
Evalstotug is a monoclonal antibody drug targeting CTLA4 that is being developed by BioAtla, Inc. for the treatment of various neoplastic and digestive, respiratory, skin, musculoskeletal, and urogenital disorders. Its highest phase of development is Phase 1/2 globally, with pending status in China, indicating its potential as a novel therapeutic option for a range of serious medical conditions.