Coherus Reveals Initial Findings from Phase I Dose-escalation Trial of its CCR8-targeting Antibody, CHS-114
Coherus BioSciences, Inc. presented results from the CHS-114 monotherapy dose escalation part of its Phase 1 trial at the ASCO Annual Meeting, held from May 31 to June 4, 2024, at McCormick Place in Chicago. CHS-114 is an innovative afucosylated human immunoglobulin G1 monoclonal antibody designed to selectively and effectively target human CCR8 without any off-target interactions.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
CCR8 is a G protein-coupled receptor that is primarily expressed on tumor-resident Treg cells, showing potential as a therapeutic target for selectively modulating immune suppression within the tumor microenvironment without causing widespread depletion of Treg cells, which can lead to undesirable autoimmune responses.
"The preliminary results from the Phase 1 dose escalation study mark a significant achievement in advancing our innovative immuno-oncology pipeline. We are greatly satisfied with the safety profile, the predictable dose-dependent pharmacokinetic behavior, and the specific depletion of peripheral CCR8+ Tregs observed," stated Rosh Dias, M.D., Chief Medical Officer of Coherus.
"By targeting CCR8, we believe CHS-114 could potentially mitigate Treg-mediated immune suppression within the TME, facilitating T cell infiltration, thereby converting cold tumors into hot ones and enhancing anti-tumor activity when combined with immuno-oncology agents. This data supports further investigation of CHS-114 in combination with our anti-PD-1 antibody, toripalimab, and other immuno-oncology therapies," added Rosh Dias.
CCR8 is a chemokine receptor predominantly found on tumor-infiltrating Tregs that diminish the body's natural anti-cancer immune response. Targeting CCR8 represents a promising therapeutic strategy aimed at selectively depleting intratumoral CCR8+ Tregs, modifying the tumor microenvironment by reducing local immunosuppression, and boosting the anti-tumor immune response when used alongside immuno-oncology agents. Data presented at ASCO confirm the mechanism of selective CCR8+ Treg depletion and show an acceptable safety profile thus far.
CHS-114, a human afucosylated anti-CCR8 monoclonal antibody, is designed to specifically target human CCR8 and preferentially deplete CCR8+ Tregs within the tumor microenvironment while sparing effector T cells in tumors or Tregs in normal tissues. In preclinical trials, CHS-114 induced antibody-dependent cellular cytotoxicity and/or antibody-dependent cellular phagocytosis to deplete tumoral CCR8+ Tregs.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
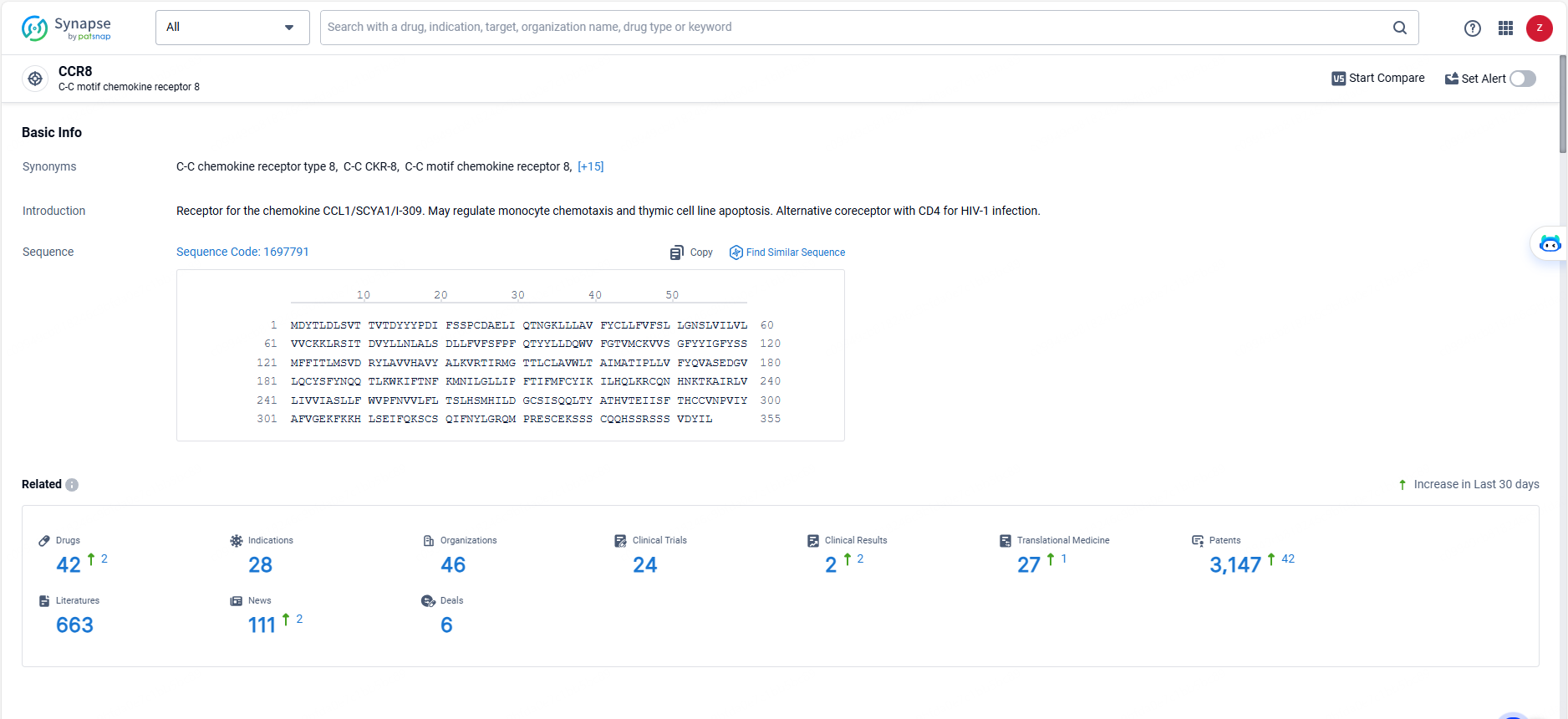
According to the data provided by the Synapse Database, As of May 29, 2024, there are 42 investigational drugs for the CCR8 target, including 28 indications, 46 R&D institutions involved, with related clinical trials reaching 24, and as many as 3147 patents.
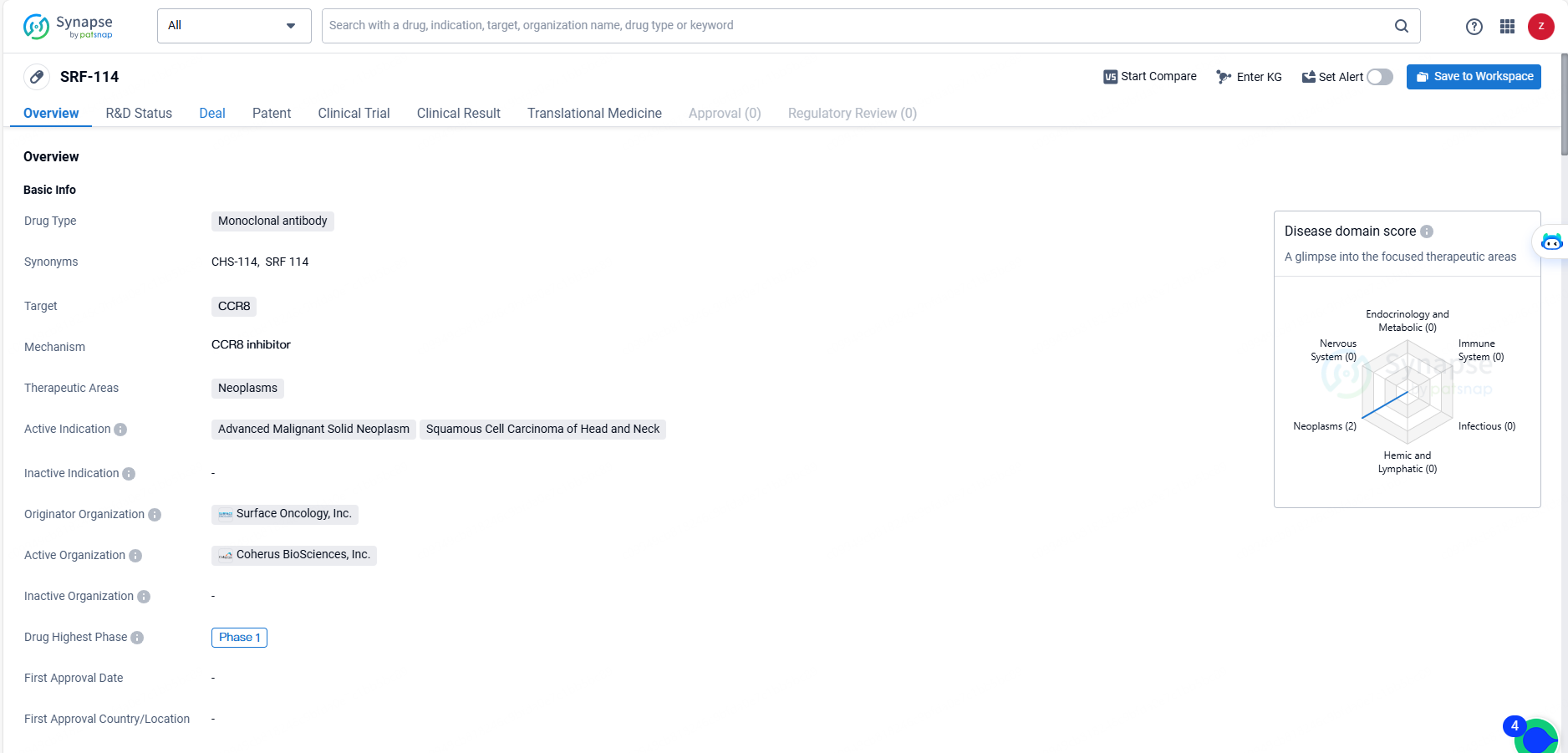
SRF-114 is a monoclonal antibody drug targeting CCR8, with a focus on treating neoplasms, particularly advanced malignant solid neoplasms and squamous cell carcinoma of the head and neck. Its current status at Phase 1 indicates that it has entered clinical testing in humans, marking an important milestone in its development as a potential therapeutic option for cancer patients.