BioCity's SC0062, a Selective Endothelin Receptor A Antagonist, Meets Main Goal in Phase 2 IgA Nephropathy Trial
BioCity Biopharma reported that its selective endothelin receptor type A (ETA) antagonist, SC0062, achieved the primary goal of reducing proteinuria in the 2-SUCCEED trial. This trial is a phase 2 clinical study that is randomized, double-blind, placebo-controlled, and dose-ranging, focusing on SC0062 in patients with chronic kidney disease. The research is still ongoing within the diabetic kidney disease group.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
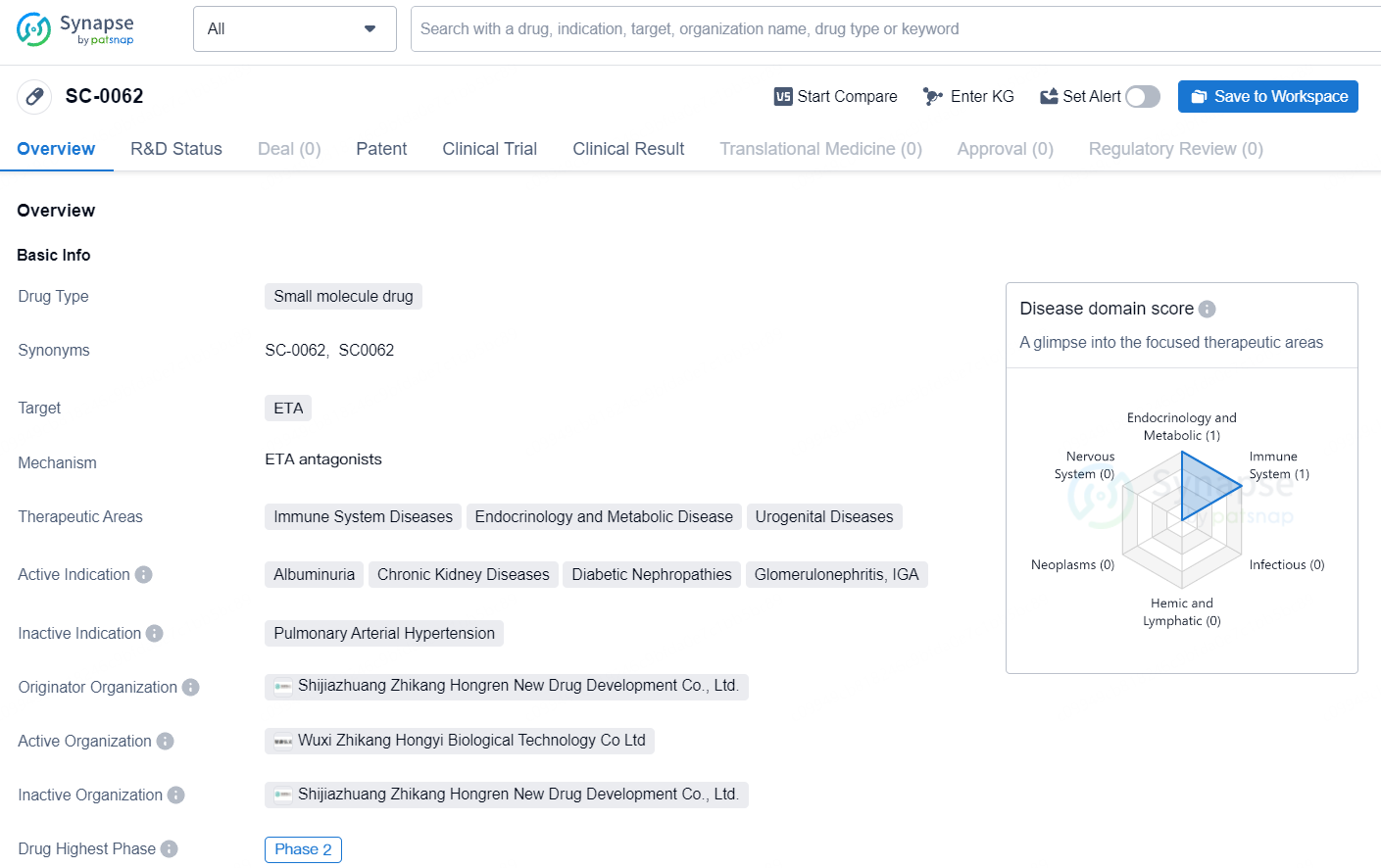
SC0062 is a new, highly selective endothelin receptor A antagonist aimed at treating CKD, known for enhancing renal blood flow, lowering proteinuria, and mitigating inflammation and fibrosis. SC0062 has been meticulously crafted to balance efficacy and safety, specifically to avoid fluid retention—a common adverse effect seen with less selective ET antagonists due to unwanted endothelin receptor B (ETB) inhibition.
Dr. Hiddo Lambers Heerspink, renowned for his expertise in CKD clinical trials, commented post-review, “The SC0062 data are impressive, particularly the evident dose-response relationship, the significant reduction in proteinuria, and the absence of peripheral edema. I am eager to collaborate with BioCity to further develop SC0062 and bring this selective endothelin receptor antagonist to CKD patients.”
Dr. Ivy Wang, Co-founder, CSO, and EVP of BioCity, added, “Recent research indicates that a molecule with increased selectivity for ETA reduces adverse effects such as sodium retention caused by ETB inhibition and is likely to enhance therapeutic outcomes. SC0062, showcasing high ETA selectivity, has shown exceptional safety and efficacy in the 2-SUCCEED study, validating our molecular design hypothesis. We are committed to offering global CKD patients safer, more effective, and reliable long-term therapeutic options, and this successful outcome fuels our determination to further this project.”
Dr. Yong Jiang Hei, CEO of BioCity, expressed, “I’m thrilled about the promising clinical trial results of SC0062, which could emerge as a significant treatment for CKD—a condition with substantial unmet medical needs. SC0062, with its high selectivity for ETA over endothelin receptor B, indicates a greater potential to curb CKD progression while avoiding safety issues linked to non-selective ET antagonists.
Preclinical studies revealed that SC0062 ameliorates pathological scores in acute kidney injury and CKD models. Phase I trials affirmed SC0062’s favorable safety profile, good tolerability, and predictable pharmacokinetics. No fluid retention was observed in either healthy volunteer trials or the IgAN cohort within the Phase II study, suggesting SC0062 may be a best-in-class ETA selective antagonist.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
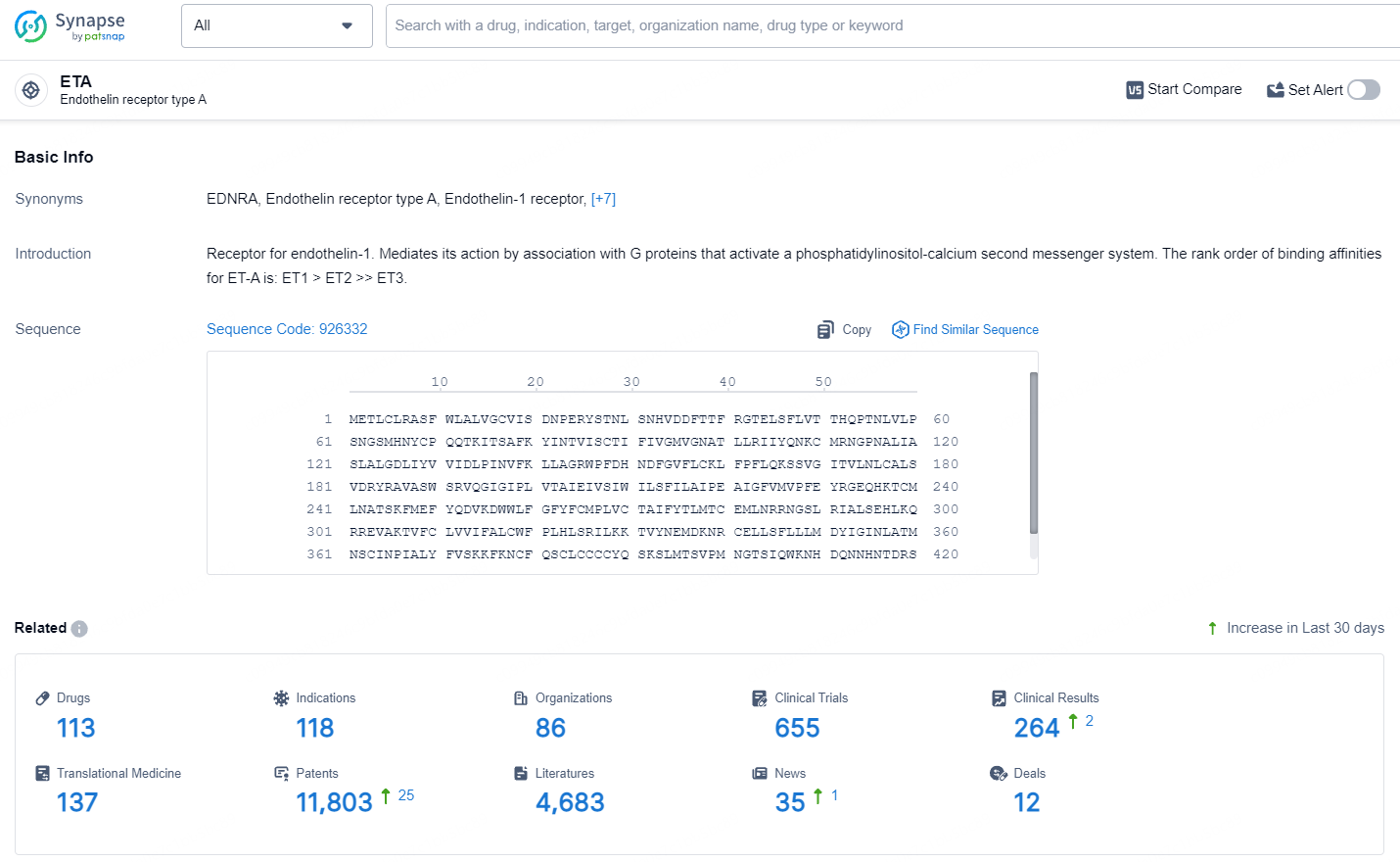
According to the data provided by the Synapse Database, As of July 9, 2024, there are 113 investigational drugs for the ETA target, including 118 indications, 86 R&D institutions involved, with related clinical trials reaching 655, and as many as 11803 patents.
SC-0062 targets ETA and is being developed for the treatment of immune system diseases, endocrinology and metabolic diseases, and urogenital diseases. SC-0062 represents a significant advancement in the field of biomedicine, particularly in the areas of immune system diseases, endocrinology and metabolic diseases, and urogenital diseases. The drug has the potential to address unmet medical needs in conditions such as chronic kidney diseases, diabetic nephropathies, and glomerulonephritis.