Is Vericiguat approved by the FDA?
Vericiguat, marketed under the brand name Verquvo, is an oral medication belonging to the drug class of vasodilators. Vericiguat received approval from the U.S. Food and Drug Administration (FDA) on January 19, 2021. This approval provides a new therapeutic option for patients with symptomatic chronic heart failure and reduced ejection fraction, aiming to reduce the risk of cardiovascular death and heart failure hospitalization after a recent hospitalization or outpatient intravenous diuretic treatment.
Uses and Administration
Uses:
- Vericiguat is indicated for adults with symptomatic chronic heart failure and an ejection fraction of less than 45%.
- It helps to lower the risk of cardiovascular death and the need for hospitalization due to heart failure exacerbations.
Administration:
- Vericiguat is taken orally once a day with food.
- The initial dose is typically 2.5 mg, which may be doubled approximately every two weeks to reach the target dose of 10 mg daily, depending on the patient's tolerance.
- If a tablet cannot be swallowed whole, it may be crushed and mixed with water, to be consumed immediately.
Precautions and Considerations
Before Taking Vericiguat:
- Patients should inform their doctor of any allergies or adverse reactions to medications.
- Vericiguat should not be used by patients who are pregnant due to the risk of birth defects. Effective birth control should be used during treatment and for at least one month after the last dose.
- Breastfeeding is not recommended during treatment with vericiguat.
Potential Interactions:
- Vericiguat should not be used in conjunction with similar medications such as riociguat.
- Patients should discuss all other medications, including over-the-counter drugs, vitamins, and herbal supplements, with their healthcare provider to avoid potential interactions.
Side Effects
Common Side Effects:
- Anemia
- Low blood pressure
Serious Side Effects:
- Light-headedness or feeling faint
- Signs of low red blood cells such as pale skin, unusual tiredness, feeling light-headed, shortness of breath, or cold hands and feet
Patients experiencing serious side effects should seek immediate medical attention. For any concerns about side effects, contacting a healthcare provider is recommended, and adverse effects can be reported to the FDA at 1-800-FDA-1088.
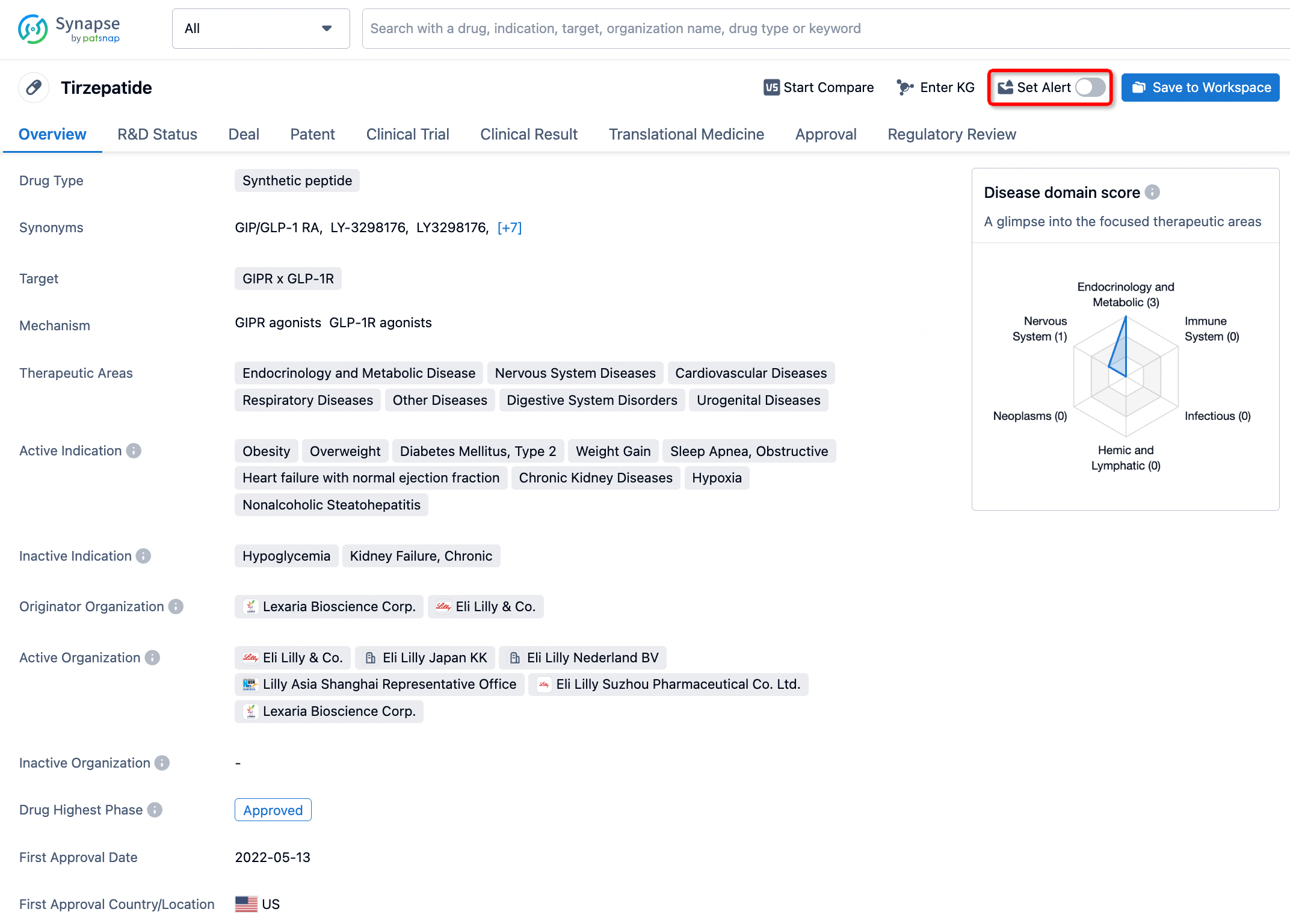
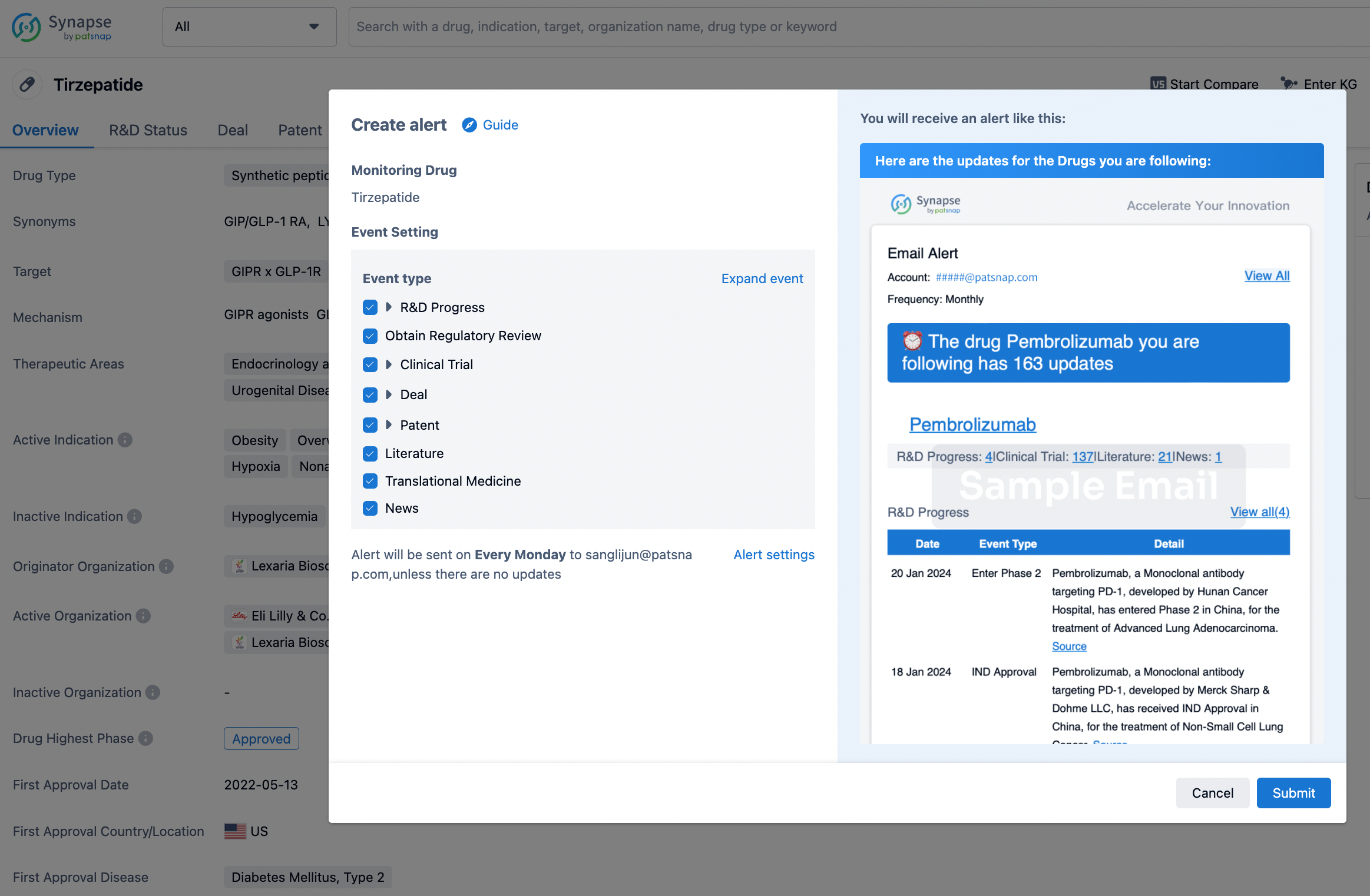
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!