Biogen's tau-targeted antisense oligonucleotide therapy shows positive clinical data in the treatment of Alzheimer's disease
Recently, Biogen announced positive results from the Phase 1b trial of BIIB080, an investigational antisense oligonucleotide (ASO) therapy targeting tau protein for the treatment of mild Alzheimer's Disease (AD). Analysis showed that patients treated with the therapy demonstrated a trend of improvement in cognitive decline. This is the first tau protein-targeting drug shown to reduce tau protein aggregation and exhibit a positive trend in patient outcomes.
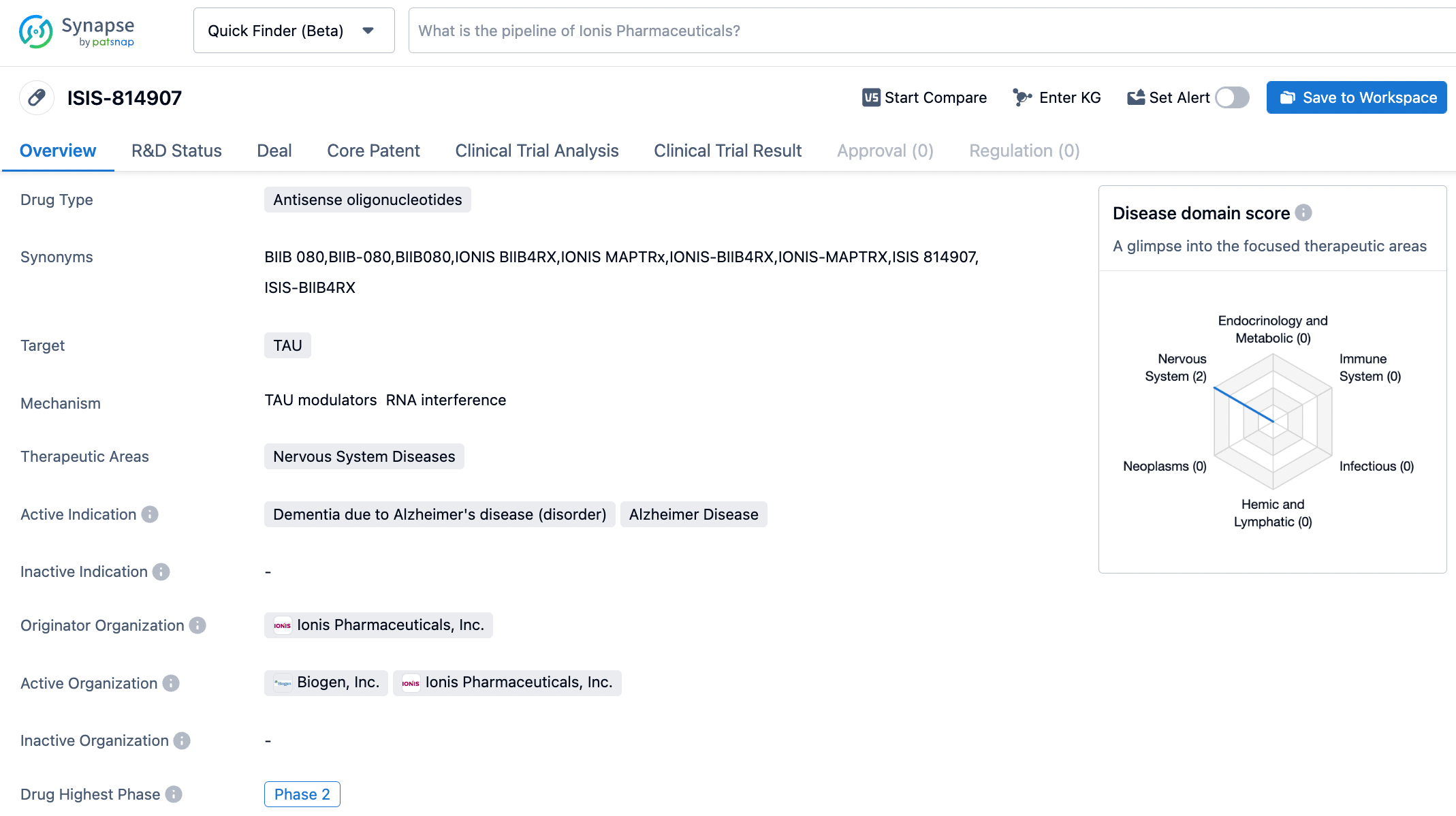
BIIB080 (ISIS-814907) was initially discovered by Ionis Pharmaceuticals as an investigational ASO therapy aimed at targeting the mRNA of microtubule-associated protein Tau (MAPT) to prevent the production of tau protein. In December 2019, Biogen and Ionis exercised a licensing option, securing an exclusive global patent fee license agreement to develop and commercialize BIIB080 (tau ASO).
The data released showed that BIIB080 reduced the concentration of soluble tau protein in the cerebrospinal fluid (CSF) of patients with early-stage Alzheimer's disease, a result that was sustained and dose-dependent. After evaluating brain composites with positron emission tomography (PET), BIIB080 was also found to reduce aggregated pathological tau protein. According to public information, BIIB080 is the first tau protein-targeting drug shown to reduce tau protein aggregation and show a positive trend in patient outcomes. Currently, the phase II clinical trial of the drug is underway.
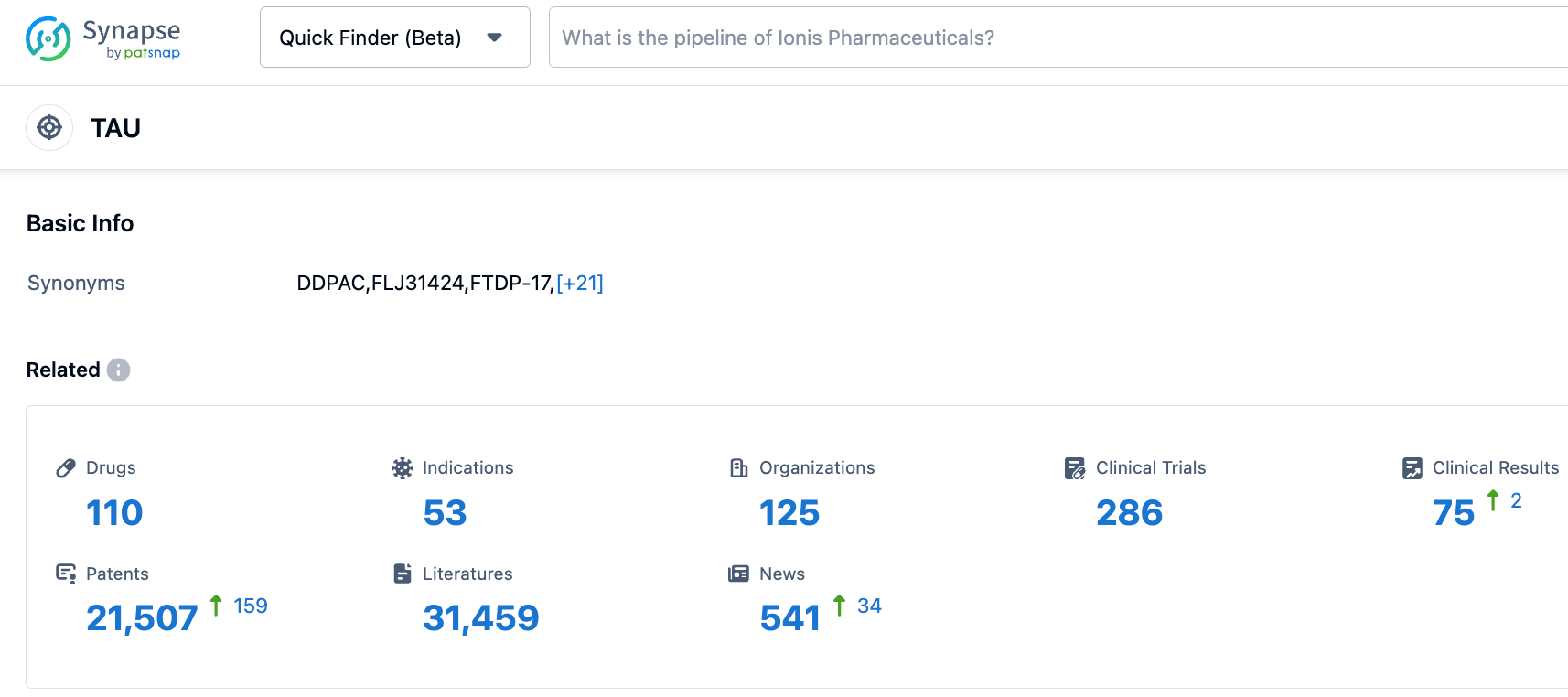
According to information disclosed by the Synapse database, as of October 27, 2023, there are 110 investigational drugs targeting Tau, with 53 indications, 125 institutions involved, 286 related clinical trials, and as many as 21,472 patents... Biogen's marketed drug Spinraza has already proven the commercial value of ASO drugs, even achieving impressive market performance in the field of rare diseases with fewer patients. We look forward to the successful development of this tau-targeted antisense oligonucleotide therapy, BIIB080.