Request Demo
Last update 06 Nov 2025
ISIS-814907
Last update 06 Nov 2025
Overview
Basic Info
Drug Type ASO |
Synonyms IONIS BIIB4RX, IONIS MAPTRx, IONIS-BIIB4RX + [6] |
Target |
Action inhibitors |
Mechanism TAU inhibitors(Microtubule-associated protein tau inhibitors) |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization |
Drug Highest PhasePhase 2 |
First Approval Date- |
RegulationFast Track (United States) |
Login to view timeline
Structure/Sequence
Boost your research with our RNA technology data.
login
or
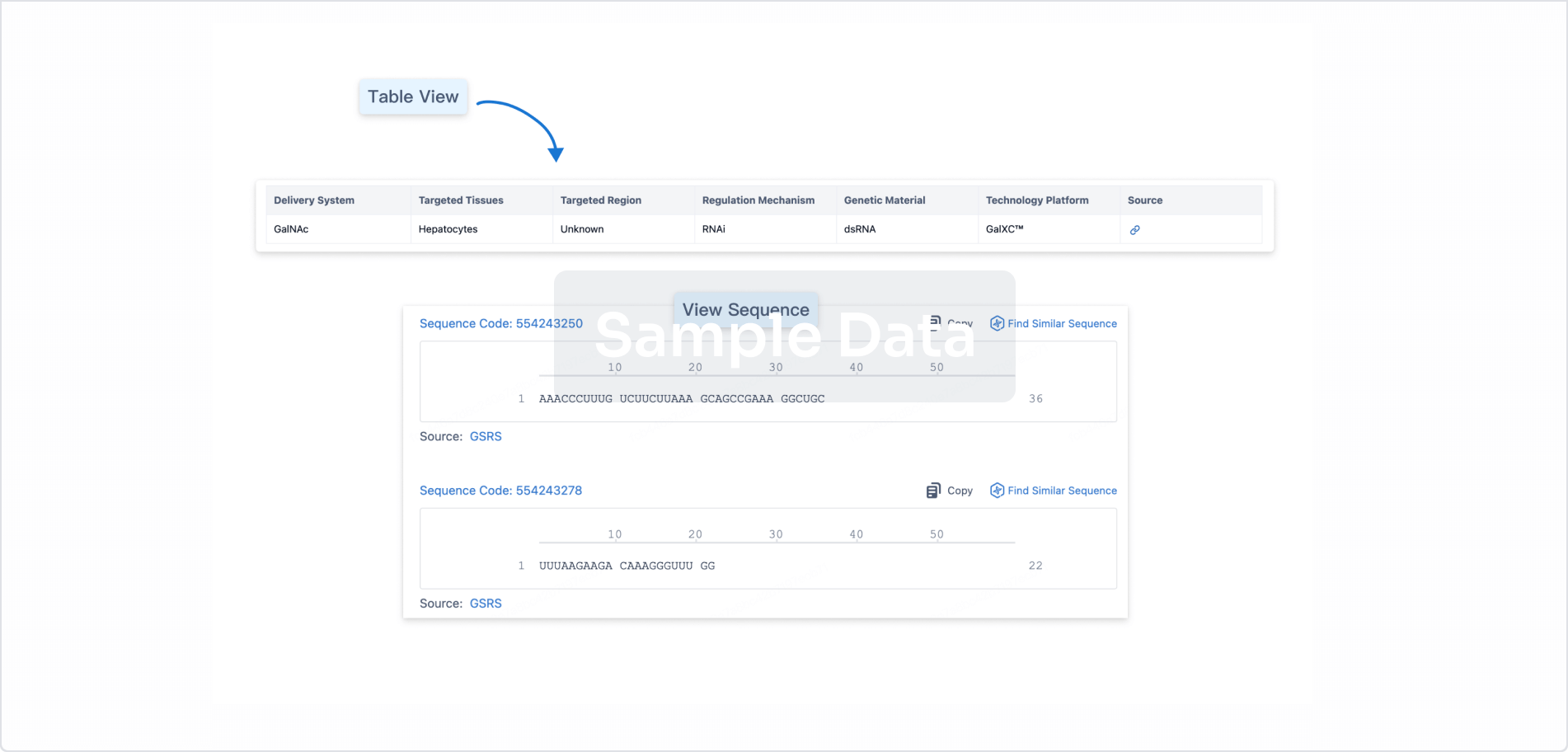
Sequence Code 539537227

Source: *****
Related
5
Clinical Trials associated with ISIS-814907NCT06454721
A Phase 1, Open-Label Study to Determine the Biodistribution, Safety, and Tolerability of a Microdose of Radiolabeled BIIB080 Co-administered With BIIB080 in Healthy Adults
In this study, researchers will learn more about a study drug called BIIB080. BIIB080 is currently a drug under investigation for treatment of Alzheimer's disease. The main question researchers are trying to answer in this study is how radiolabeled BIIB080 distributes in the brain and spinal cord. To help answer this question, researchers will use positron emission tomography (PET) scanner that can detect radiolabeled BIIB080 after a single injection of a small dose of radiolabeled BIIB080 ([89Zr]Zr-DFO-BIIB080) and a dose of BIIB080 together via an intrathecal (IT) injection in healthy volunteers. Researchers will also learn about the safety of injecting radiolabeled BIIB080 and BIIB080 together.
Start Date30 Jan 2025 |
Sponsor / Collaborator |
NL-OMON57028
Moses II - Measuring Elecsys β-Amyloid (1-42) II and Phospho-Tau (181P) in CSF Samples supporting Phase 2 Clinical Trial of 247AD201 (BIIB080) - Moses II
Start Date21 Feb 2023 |
Sponsor / Collaborator |
NCT05399888
A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Assess the Efficacy, Safety, and Tolerability of BIIB080 in Subjects With Mild Cognitive Impairment Due to Alzheimer's Disease or Mild Alzheimer's Disease Dementia
In this study, researchers will learn more about a study drug called BIIB080. The study will focus on participants with mild cognitive impairment or mild dementia due to AD.
The main question researchers are trying to answer is if BIIB080 can slow the worsening of AD more than placebo. It will focus on what dose of BIIB080 slows worsening of AD the most.
To help answer this question, researchers will use the Clinical Dementia Rating-Sum of Boxes, also known as the CDR-SB.
* Clinicians use the CDR-SB to measure several categories of dementia symptoms.
* The results for each category are added together for a total score. Lower scores are better.
Researchers will also learn more about the safety of BIIB080.
The study will be split into 2 parts. The 1st part is the Placebo-Controlled Period. The 2nd part is the Long-Term Extension (LTE) Period. The 2nd part of the study will help researchers learn about the long-term safety of BIIB080, and how it affects the participant's daily life, thinking, and memory abilities in the longer term.
A description of how the study will be done is given below.
* After screening, participants will first receive either a low dose or high dose of BIIB080, or a placebo, as an injection into the fluid around the spinal cord (cerebrospinal fluid). A placebo looks like the study drug but contains no real medicine.
* Participants will receive BIIB080 or placebo once every 12 weeks or 24 weeks.
* After 76 weeks of treatment in the Placebo-Controlled Period, eligible participants will move onto the Extension Treatment period, which will last 96 weeks.
* In the extension period, participants who received placebo will be switched to high dose BIIB080 every 12 or 24 weeks.
* Participants may be in the study for up to 201 weeks, or about 4 years. This includes the screening and follow-up periods.
* Participants can continue to take certain medications for AD. Participants must be on the same dose of medication for at least 8 weeks before the screening period.
* After the screening period, most participants will visit the clinic every 6 weeks.
The main question researchers are trying to answer is if BIIB080 can slow the worsening of AD more than placebo. It will focus on what dose of BIIB080 slows worsening of AD the most.
To help answer this question, researchers will use the Clinical Dementia Rating-Sum of Boxes, also known as the CDR-SB.
* Clinicians use the CDR-SB to measure several categories of dementia symptoms.
* The results for each category are added together for a total score. Lower scores are better.
Researchers will also learn more about the safety of BIIB080.
The study will be split into 2 parts. The 1st part is the Placebo-Controlled Period. The 2nd part is the Long-Term Extension (LTE) Period. The 2nd part of the study will help researchers learn about the long-term safety of BIIB080, and how it affects the participant's daily life, thinking, and memory abilities in the longer term.
A description of how the study will be done is given below.
* After screening, participants will first receive either a low dose or high dose of BIIB080, or a placebo, as an injection into the fluid around the spinal cord (cerebrospinal fluid). A placebo looks like the study drug but contains no real medicine.
* Participants will receive BIIB080 or placebo once every 12 weeks or 24 weeks.
* After 76 weeks of treatment in the Placebo-Controlled Period, eligible participants will move onto the Extension Treatment period, which will last 96 weeks.
* In the extension period, participants who received placebo will be switched to high dose BIIB080 every 12 or 24 weeks.
* Participants may be in the study for up to 201 weeks, or about 4 years. This includes the screening and follow-up periods.
* Participants can continue to take certain medications for AD. Participants must be on the same dose of medication for at least 8 weeks before the screening period.
* After the screening period, most participants will visit the clinic every 6 weeks.
Start Date24 Aug 2022 |
Sponsor / Collaborator |
100 Clinical Results associated with ISIS-814907
Login to view more data
100 Translational Medicine associated with ISIS-814907
Login to view more data
100 Patents (Medical) associated with ISIS-814907
Login to view more data
2
Literatures (Medical) associated with ISIS-81490707 May 2025·Science Translational Medicine
PET imaging of antisense oligonucleotide distribution in rat and nonhuman primate brains using click chemistry
Article
Author: Amatruda, Mario ; DuBois, Jonathan M. ; Jiang, Di ; Peterson, Emily A. ; Datta, Prodip ; Girmay, Sara ; Delavari, Armin ; Boscutti, Giulia ; Halldin, Christer ; Forsberg Morén, Anton ; Muste, Cathy ; Klein, Stephanie K. ; Kaliszczak, Maciej ; Ochniewicz, Piotr ; Plisson, Christophe ; Tang, Sac-Pham ; Nag, Sangram ; Martarello, Laurent ; Pickel, Thomas C. ; Yuan, Long ; Zhang, Qize ; Khani Meynaq, Yasir ; Beshr, Rouaa ; Cook, Brendon E. ; Bolduc, Philippe N. ; Polyak, Ildiko ; Shoroye, Adebowale
Determination of a drug’s biodistribution is critical to ensure that it reaches the target tissue of interest. This is particularly challenging in the brain, where invasive sampling methods may not be possible. Here, we present a pretargeted positron emission tomography (PET) imaging methodology that uses bioorthogonal click chemistry to determine the distribution of an antisense oligonucleotide (ASO) in the brains of rats and nonhuman primates after intrathecal dosing of ASO. A PET tracer, [
18
F]BIO-687, bearing a click-reactive
trans
-cyclooctene was developed and tested in conjunction with a test Malat1 ASO conjugated with a methyltetrazine group. PET imaging in rats demonstrated that the tracer had good kinetic properties for PET imaging in the rodent central nervous system and could react to form a covalent linkage with high specificity to the methyltetrazine-conjugated ASO in vivo. Furthermore, the amount of PET tracer reacted by cycloaddition with the methyltetrazine was determined to be dependent on the concentration of ASO-methyltetrazine in rat brain tissue, as determined by comparing the PET imaging signal with the liquid chromatography–mass spectrometry signal in the tissue homogenates. The approach was evaluated in cynomolgus macaques using both the Malat1 test ASO and a candidate therapeutic ASO, BIIB080, targeting the microtubule-associated protein tau (
MAPT
) gene. PET imaging showed favorable tracer kinetics and specific binding to both ASOs in nonhuman primate (NHP) brain in vivo. These results suggest that the PET imaging tracer [
18
F]BIO-687 could show the distribution of intrathecally delivered ASOs in the rat and NHP brains.
01 Dec 2023·JAMA neurology
Exploratory Tau Biomarker Results From a Multiple Ascending-Dose Study of BIIB080 in Alzheimer Disease
Article
Author: Kordasiewicz, Holly ; Martarello, Laurent ; Wu, Shuang ; Mignon, Laurence ; Ziogas, Nick ; Hutchison, R. Matthew ; Beaver, John ; Budd Haeberlein, Samantha ; Li, Yumeng ; Lin, Lin ; DuBois, Jonathan ; Lane, Roger ; Edwards, Amanda L. ; Junge, Candice ; Collins, Jessica A. ; Shulman, Melanie ; Graham, Danielle
Importance:
Accumulation of hyperphosphorylated, tangled microtubule-associated protein tau (MAPT) is a pathological hallmark of Alzheimer disease (AD) associated with disease progression and cognitive decline.
Objective:
To evaluate the effect of tau synthesis reduction on tau biomarkers in patients with mild AD.
Design, Setting, and Participants:
This randomized clinical trial was a double-blind, placebo-controlled 36-week multiple-ascending dose (MAD) phase 1b trial (October 2017 to September 2020), followed by a 64- or 71-week open-label long-term extension (LTE) (October 2019 to May 2022). After being assessed for eligibility at 12 sites in Canada and Europe, participants with mild AD and confirmed amyloid pathology were randomized 3:1 (BIIB080:placebo) in 4 dose cohorts.
Intervention:
Intrathecal administration of BIIB080, a MAPT-targeting antisense oligonucleotide, or placebo. Active dose arms included 10 mg every 4 weeks, 30 mg every 4 weeks, 60 mg every 4 weeks, and 115 mg every 12 weeks during the MAD period and 60 mg every 12 weeks or 115 mg every 12 weeks during the LTE.
Main Outcome and Measures:
The original primary end point was safety. Additionally, BIIB080, total tau (t-tau), and phosphorylated tau 181 (p-tau181) cerebrospinal fluid (CSF) concentrations were evaluated. Tau positron emission tomography (PET) was collected in a substudy, and standard uptake value ratios (SUVRs) were calculated in a priori-defined composite regions of interest.
Results:
Of 102 participants assessed for eligibility, 46 participants with mild AD were enrolled; 23 (50%) were female, and mean (SD) age was 65.8 (5.70) years. BIIB080 was generally well tolerated and was associated with a dose-dependent reduction in CSF t-tau and p-tau181 in the MAD period (56% reduction; 95% CI, 50% to 62%; and 51% reduction; 95% CI, 38% to 63%, of CSF t-tau in the 2 higher-dose cohorts) that continued and/or was maintained through quarterly dosing in the LTE. Tau PET demonstrated reduced accumulation vs placebo at week 25 (n = 13). At week 100, tau PET showed a reduction from baseline across all regions assessed (n = 12), with the largest reductions from baseline observed in the temporal composite (−0.71 SUVR; 95% CI, −1.40 to −0.02). A moderate correlation was observed between model-predicted cumulative CSF drug exposure and tau PET change.
Conclusions and Relevance:
In this randomized clinical trial, BIIB080 reduced tau biomarkers, including CSF t-tau, CSF p-tau181, and tau PET, which is associated with cognitive decline, in participants with mild AD. Effects of BIIB080 on biomarkers and clinical outcomes are being further evaluated in a phase 2 trial.
Trial Registration:
ClinicalTrials.gov Identifier: NCT03186989
46
News (Medical) associated with ISIS-81490727 Oct 2025
None
Alfred W. Sandrock, Jr., M.D., Ph.D.Chief Executive Officer of Voyager TherapeuticsAs the annual Clinical Trials on Alzheimer’s Disease (CTAD) conference approaches, new data on emerging targets such as tau and advanced delivery methods are converging.Researchers in the field of Alzheimer’s Disease (AD) are not known for optimism. We had decades with precious little to be optimistic about; decades in which one seemingly promising drug after another failed.Recently, the momentum in AD has begun to shift. We now have two FDA-approved drugs, lecanemab (Leqembi) and donanemab (Kisunla), shown to slow disease progression and cognitive and functional decline in some patients. Both drugs are anti-amyloid antibodies, and critics note that they are only about 30% effective. I would remind those critics that early drugs for multiple sclerosis were also only about 30% effective, while today’s MS drugs hit 60-70%; you have to start somewhere. In AD and neurodegenerative diseases in general, what has been particularly exciting is the speed at which human genetics and advances in brain imaging are helping us understand the molecular underpinnings of the disease – enabling us to know not only what to target, but how to reach it and how to measure the impact. Case in point is the explosion of interest around tau.The Emergence of Tau: the bullet causing neurodegeneration in ADWhile amyloid dominated AD research for decades, tau is emerging as the next critical target. Recent data have shown that the spread of pathological forms of tau protein in the brain is more closely correlated with the progression of dementia than amyloid. Increasing deposits of amyloid seem to cause tau to spread, and that spread of tau causes neurodegeneration. I often think of amyloid as the trigger, but tau as the bullet.Some of the most encouraging data on targeting tau to-date have come from Biogen’s BIIB080, an antisense oligonucleotide that, in an early clinical trial, robustly lowered tau burden as shown by tau PET imaging and resulted in favorable trends on cognition and function. Then at CTAD 2024, UCB made tau history by establishing with bepranemab that an antibody can inhibit the spread of pathological tau in the brain and slow cognitive decline – though the trial ultimately missed its primary endpoint on functional improvement.Large Phase II trials on Biogen’s BIIB080 and Johnson & Johnson’s anti-tau antibody posdinemab are expected next year. But in the interim, data on several other tau targeting programs are slated for presentation at CTAD this December, including Novartis’s NIO752, UCB’s bepranemab, Bristol Myers Squibb’s BMS-986446, Adel’s ADEL-Y01, and Voyager’s own VY7523. The Voyager team will continue to learn from the field as we complete our clinical trial of our anti-tau antibody VY7523, for which we expect tau PET imaging data next year, and prepare to move our tau silencing gene therapy VY1706 into the clinic next year.Brain-Targeted Delivery: The Key to Unlock AD Treatments?As exciting as the approved anti-amyloid and emerging anti-tau antibodies are, the hard truth remains that antibodies don’t get into the brain particularly well: on average only about 0.1% to 0.5% of the antibody gets into the brain. Fortunately, antibodies are so potent that this miniscule amount is enough to make a therapeutic difference – but no one can call it efficient.Delivery to the brain has long been the white whale of neurology drug development: the blood-brain barrier keeps out most large molecule drugs. Improving delivery could increase efficacy through improved brain targeting, enhance safety by reducing peripheral exposure, and potentially lower costs by reducing required doses.At Voyager we, and companies across the industry, are prioritizing delivery platforms to unlock the therapeutic potential of multiple modalities in neurological diseases. Voyager’s TRACER™ AAV capsids are designed to enable gene therapies for neurological diseases by providing high brain penetration following intravenous dosing. We are using this delivery approach for VY1706, our tau silencing gene therapy, as well as for an APOE gene therapy program for AD and multiple other neurology programs.Another emerging area of interest in brain-targeted delivery is “shuttles,” which leverage receptors on the blood-brain barrier to carry large molecules across it. At CTAD, Roche will share data on trontinemab, a shuttled anti-amyloid antibody. Unshuttled, the antibody (gantenerumab) worked in fewer than 30% of patients and had about a 30% rate of a dangerous side effect, ARIA-E. Trontinemab, which uses a transferrin receptor shuttle to target the antibody to the brain, has shown effectiveness in 91% of patients and reduced ARIA-E to below 5%. Recently Voyager unveiled initial data with our Voyager NeuroShuttle™, which builds on first-generation transferrin receptor shuttles by leveraging a different receptor, ALPL, which has been integral in our gene therapy work. Initial preclinical data showed a distinct pharmacokinetic profile, with brain uptake sustained for greater than 3 weeks post-dose, versus less than one week for transferrin shuttles.More Voyager NeuroShuttle data are on the way – though not in time for CTAD 2025. Even so, the data emerging across the industry on tau-targeted therapies, biomarkers, and next-generation delivery platforms give me optimism – a rare gift for an AD researcher.
Gene TherapyOligonucleotidePhase 2
21 Jul 2025
Lecanemab presentations to include long-term Clarity AD data, real-world treatment insights, and a subcutaneous formulation for continued care Presentations on tau to explore its biological role, the development of targeted therapies and biomarkers, and the future integration of these innovations into clinical practice
CAMBRIDGE, Mass., July 21, 2025 (GLOBE NEWSWIRE) -- Biogen Inc. (Nasdaq: BIIB) today announced upcoming scientific presentations at the 2025 Alzheimer’s Association International Conference (AAIC), taking place July 27-31 in Toronto, Canada. Data on LEQEMBI® (lecanemab) will include 48-month results from the Clarity AD open-label extension, real-world evidence, and new insights into a subcutaneous formulation for maintenance dosing. Presentations on tau will explore tau-targeted therapies and biomarkers, including baseline characteristics of participants from CELIA, a Phase 2 trial evaluating the efficacy, safety, and tolerability of BIIB080, an investigational antisense oligonucleotide (ASO) therapy that targets tau.
“At AAIC, we are sharing data that underscore our ongoing efforts to advance both how Alzheimer’s is treated and how care is delivered, including 48-month findings from the LEQEMBI Clarity AD open-label extension and new insights into the potential of subcutaneous maintenance dosing for LEQEMBI. We are also excited to share baseline characteristics from CELIA, our Phase 2 study of BIIB080, an investigational ASO therapy targeting tau,” said Priya Singhal, M.D., M.P.H., Head of Development at Biogen. “As we deepen our understanding of this complex disease, we remain committed to pushing the science forward and evolving care to better meet the needs of patients and families.”
Key Scientific Sessions and Presentations:
Lecanemab Clarity AD OLE in Early AD: Initial Findings from 48-Month AnalysisWednesday, July 30, 8:00–8:45 AM ET Lecanemab Subcutaneous Formulation for Maintenance Dosing: The Potential of a New and Convenient Option for Ongoing Treatment in Early Alzheimer’s DiseaseWednesday, July 30, 9:00–10:30 AM ET Patient, Care Partner, and Health Care Professional Opinion of the Lecanemab Autoinjector for Subcutaneous DeliverySunday, July 27, 8:00–8:45 AM ET Lecanemab Two Years Post-Approval: Real-World Case Series and Patient Pathway LearningsSunday, July 27, 9:00–10:30 AM ET Indirect Treatment Comparison of ARIA Outcomes for Lecanemab Compared to Donanemab Based on Reported ResultsSunday, July 27, 4:14–5:45 PM ET Innovations in Tau Therapies and Biomarkers for Alzheimer’s Disease: Bridging Research and Clinical PracticeWednesday, July 30, 2:00–3:30 PM ET Baseline Characteristics from CELIA: A Phase 2 Study to Evaluate BIIB080 in Participants with Early Alzheimer’s DiseaseMonday, July 28, 7:30 AM–4:15 PM ET
Educational Program on Tau in Alzheimer’s Disease At AAIC, Biogen will host an interactive booth offering an immersive journey into the role of tau in Alzheimer’s disease, from pathology to clinical presentation. Biogen is also expanding its educational efforts with a new e-learning module on KnowTau.com, building on the resources already available.
For more information, please see the AAIC 2025 program and visit the Biogen AAIC booth.
About BIIB080BIIB080 is an investigational antisense oligonucleotide (ASO) therapy designed to target microtubule-associated protein tau (MAPT) mRNA to reduce the production of tau protein. Abnormal accumulation of tau in the brain is a hallmark of Alzheimer’s disease and is associated with neurodegeneration and cognitive decline. BIIB080 is currently being evaluated in a Phase 2 clinical study (NCT05399888) in individuals with early Alzheimer’s disease.
In December 2019, Biogen exercised a license option with Ionis Pharmaceuticals and obtained a worldwide, exclusive, royalty-bearing license to develop and commercialize BIIB080 (tau ASO). BIIB080 was discovered by Ionis.
About LEQEMBI ® (lecanemab)LEQEMBI (lecanemab) is the result of a strategic research alliance between Eisai and BioArctic. LEQEMBI is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble (protofibril) and insoluble forms of amyloid-beta (Aβ). LEQEMBI is an amyloid beta-directed antibody for the treatment for Alzheimer’s disease (AD) in the U.S. The U.S. Food and Drug Administration (FDA) granted LEQEMBI traditional approval on July 6, 2023.
LEQEMBI is indicated for the treatment of Alzheimer’s disease. Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.
Eisai and Biogen have been collaborating on the joint development and commercialization of AD treatments since 2014. Eisai serves as the lead of LEQEMBI development and regulatory submissions globally with both companies co-commercializing and co-promoting the product and Eisai having final decision-making authority.
Please see full U.S. Prescribing Information for LEQEMBI, including Boxed WARNING and Medication Guide.
About BiogenFounded in 1978, Biogen is a leading biotechnology company that pioneers innovative science to deliver new medicines to transform patients’ lives and to create value for shareholders and our communities. We apply deep understanding of human biology and leverage different modalities to advance first-in-class treatments or therapies that deliver superior outcomes. Our approach is to take bold risks, balanced with return on investment to deliver long-term growth.We routinely post information that may be important to investors on our website at www.biogen.com. Follow us on social media - Facebook, LinkedIn, X, YouTube.
Biogen Safe Harbor This news release contains forward-looking statements, including about the potential clinical effects of lecanemab and BIIB080; the potential benefits, safety and efficacy of lecanemab and BIIB080; potential regulatory discussions, submissions and approvals and the timing thereof; the treatment of Alzheimer's disease; the anticipated risks, benefits and potential of Biogen's collaboration arrangements with Eisai; the potential of Biogen's commercial business and pipeline programs, including lecanemab and BIIB080; and risks and uncertainties associated with drug development and commercialization. These forward-looking statements may be accompanied by such words as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “guidance,” “hope,” “intend,” “may,” “objective,” “plan,” “possible,” “potential,” “predict,” “project,” “prospect,” “should,” “target,” “will,” “would,” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements. Given their forward-looking nature, these statements involve substantial risks and uncertainties that may be based on inaccurate assumptions and could cause actual results to differ materially from those reflected in such statements.
These forward-looking statements are based on management's current beliefs and assumptions and on information currently available to management. Given their nature, we cannot assure that any outcome expressed in these forward-looking statements will be realized in whole or in part. We caution that these statements are subject to risks and uncertainties, many of which are outside of our control and could cause future events or results to be materially different from those stated or implied in this document, including, among others, uncertainty of long-term success in developing, licensing, or acquiring other product candidates or additional indications for existing products; expectations, plans and prospects relating to product approvals, approvals of additional indications for our existing products, sales, pricing, growth, reimbursement and launch of our marketed and pipeline products; our ability to effectively implement our corporate strategy; risks associated with third party collaborations; the risk that positive results in a clinical trial may not be replicated in subsequent or confirmatory trials or success in early stage clinical trials may not be predictive of results in later stage or large scale clinical trials or trials in other potential indications; risks associated with clinical trials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials, regulatory authorities may require additional information or further studies, or may fail to approve or may delay approval of our drug candidates; the occurrence of adverse safety events, restrictions on use with our products, or product liability claims; risks of unexpected costs or delays or other unforeseen hurdles; and any other risks and uncertainties that are described in other reports we have filed with the U.S. Securities and Exchange Commission.
These statements speak only as of the date of this press release and are based on information and estimates available to us at this time. Should known or unknown risks or uncertainties materialize or should underlying assumptions prove inaccurate, actual results could vary materially from past results and those anticipated, estimated or projected. Investors are cautioned not to put undue reliance on forward-looking statements. A further list and description of risks, uncertainties and other matters can be found in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024 and in our subsequent reports on Form 10-Q and Form 10-K, in each case including in the sections thereof captioned “Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in our subsequent reports on Form 8-K. Except as required by law, we do not undertake any obligation to publicly update any forward-looking statements whether as a result of any new information, future events, changed circumstances or otherwise.
Phase 2Clinical ResultLicense out/inDrug ApprovalOligonucleotide
10 Apr 2025
Cambridge: US-based drugmaker
Biogen
announced that the US Food and
Drug
Administration (
FDA
) has granted Fast Track designation to BIIB080, an investigational antisense oligonucleotide (ASO) therapy aimed at treating
Alzheimer
’s disease by targeting the tau protein.
BIIB080 is designed to reduce the production of microtubule-associated protein tau (MAPT) by targeting its mRNA. The candidate is currently being evaluated in a
Phase 2
clinical trial
(NCT05399888) in individuals with early-stage Alzheimer’s disease.
Priya Singhal, Head of Development, Biogen, said, “We are encouraged by the FDA’s Fast Track designation for BIIB080. As an investigational antisense therapy, it represents a differentiated approach to targeting tau and holds promising potential for patients. We are advancing this program with urgency on behalf of people living with Alzheimer’s and their families.”
Biogen acquired worldwide, exclusive, royalty-bearing rights to BIIB080 from Ionis Pharmaceuticals in December 2019, exercising a license option under their collaboration. As part of the agreement, Ionis received an upfront payment of $45 million (approx. ₹387 crore) and is eligible for up to $155 million (approx. ₹1,330 crore) in milestone payments, along with royalties in the low- to mid-teens on future sales.
Besides this candidate Biogen had collaborated with
Ionis Pharma
to acquire the global marketing rights of its SMA drug Spinraza (nusinersen) in 2016.
The Fast Track designation is intended to accelerate the development and review of investigational therapies that treat serious conditions and fulfill unmet medical needs.
By
Online Bureau
,

License out/inPhase 2OligonucleotideFast Track
100 Deals associated with ISIS-814907
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Cognitive Dysfunction | Phase 2 | United States | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Japan | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Australia | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Belgium | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Canada | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Czechia | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Denmark | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Finland | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | France | 24 Aug 2022 | |
| Cognitive Dysfunction | Phase 2 | Germany | 24 Aug 2022 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
NCT03186989 (Literature) Manual | Phase 1 | Alzheimer Disease Tau protein | 46 | ftjnycxpui(jwcwxojwjl) = fqzvjxdgbw vqpwababdr (opsmzwetlx, 50 - 62) View more | Positive | 30 Oct 2023 | |
placebo | - | ||||||
NCT03186989 (NEWS) Manual | Phase 1 | 46 | zwnxyormma(vgrblxdnvx) = fqzrkxpbpp zctifyiujx (tbsuejfwpw ) | Positive | 26 Oct 2023 | ||
NCT03186989 (PRNewswire) Manual | Phase 1 | 46 | (low dose group treated every four-weeks) | xwnzaqdisz(abneiaxich) = All adverse events were mild to moderate in severity with no serious adverse events occurring in any patients that received BIIB080. There were no deaths, dose-limiting adverse events or dosing discontinuations. uookwelfer (vgvqloxqtw ) Met | Positive | 16 Aug 2023 | |
(medium dose group treated every four-weeks) | |||||||
Phase 1 | - | hftdjimbyn(gaooelhtyq) = fgjhrbdsmu venqwenwiv (natptvuobj ) | Positive | 10 Apr 2018 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

