BioMed Pharma Concludes Initial Participant Treatment in Key Hemophilia A Study
In an official announcement, Belief BioMed Group, a front-runner in the biotechnology sector specialized in pioneering genetic treatments, disclosed the initiation of patient treatment within its pivotal clinical study for BBM-H803. This gene therapy product, targeting the treatment of hemophilia A, has been autonomously researched, devised, and manufactured by Belief BioMed.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
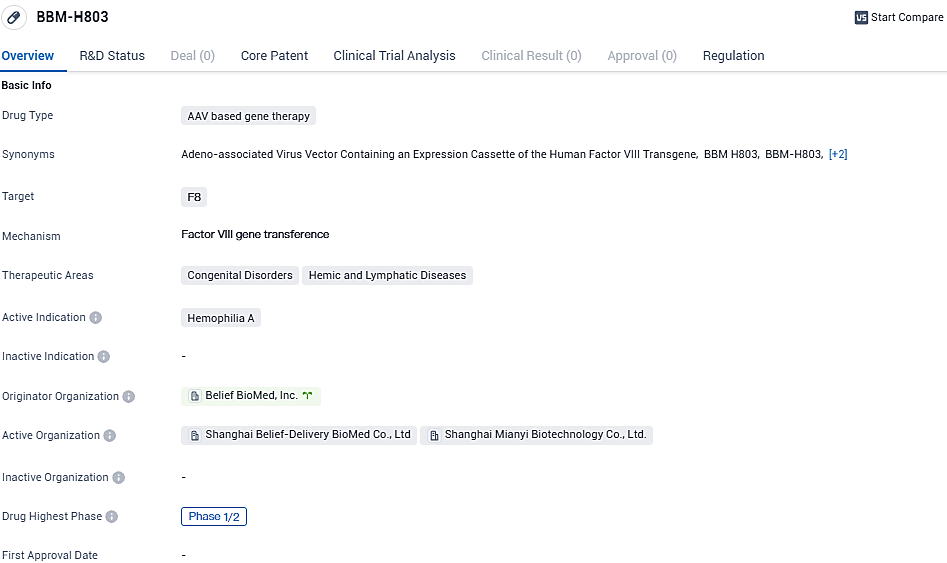
Belief BioMed's pioneering gene therapy product, BBM-H803, is aimed at treating hemophilia A and has secured its place as the organization's second medical innovation to receive the IND green light from China's National Medical Products Administration. In the final month of 2022, the U.S. FDA acknowledged BBM-H803 with the Orphan Drug status. To date, China's clinical landscape lacks adeno-associated virus (AAV) gene therapies for adult individuals diagnosed with hemophilia A.
The pivotal investigation into BBM-H803's application involves a widespread, open-label study, which engages multiple centers, operating under a single-arm directive, and focuses on a one-time injectable treatment protocol. It assesses the preparation's safety profile, its overall tolerate nature, and the effectiveness following a singular intravenous dose in candidates who are 18 years or older who present with severe hemophilia A.
BBM-H803 benefits from a proprietary capsid design, engineered by the Biomed company's team, aiming to reduce immune reactions while enhancing targeted liver uptake. Its clinical dosage recommendations are notably less than those of other international AAV gene therapy products, with an anticipated increase in patient safety as a result. Completion of participant recruitment for an earlier phase of investigative study was achieved by August 2023, with the resulting data being instrumental for advancing to the current phase of clinical trials.
Dr. Xiao Xiao, who has co-founded Belief BioMed and assumes the roles of Chairman and Chief Science Officer, remarked, "Embracing the fresh potential that the New Year unfolds, it is with immense joy that we announce a landmark achievement in this remarkable day for both the enterprise and those battling hemophilia A. The inaugural patient treatment in BBM-H803's pivotal trial has been finalized with success.
This significant progress serves as a testament to Belief BioMed’s continued advancements within the sphere of gene therapy, specifically targeting hematologic disorders, while also validating our extensive and vigorous approach to research and development. Persistent in our foundational objectives, we remain unified in our effort, both internally and with collaborative partners, to precisely and vigorously advance this clinical trial. It is with urgency and commitment that we endeavor to introduce this groundbreaking therapy to the multitude of Chinese patients in dire need."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
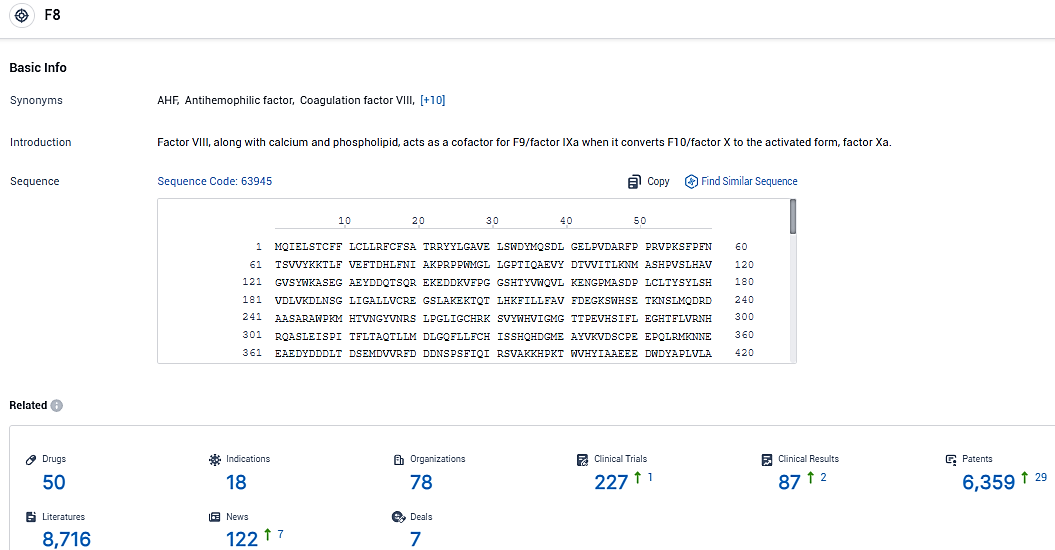
According to the data provided by the Synapse Database, As of January 6, 2024, there are 50 investigational drugs for the F8 target, including 18 indications, 78 R&D institutions involved, with related clinical trials reaching 227, and as many as 6359 patents.
BBM-H803 is an AAV-based gene therapy with independent intellectual property rights owned by Belief BioMed. It is administered intravenously to deliver the coagulation factor Ⅷ gene into the body of patients with hemophilia A, thereby improving and maintaining the coagulation factor level in the patient's body for a long time, for the prevention of bleeding. BBM-H803 was granted Orphan Drug Designation by the U.S. FDA. In May 2023, Belief BioMed submitted IND application to China NMPA, and received IND approval in July 2023.