TPO receptor agonists: What They Are and How to Stay Updated on the Latest Research
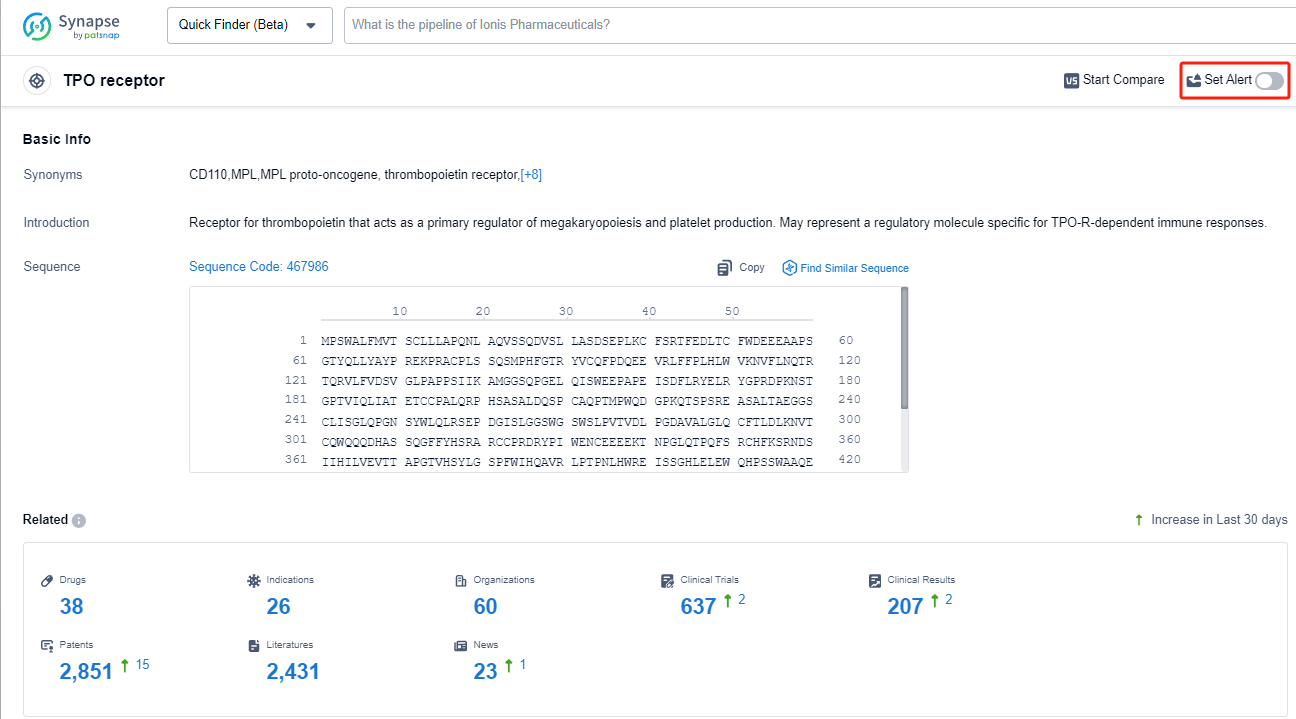
The TPO receptor, also known as the thrombopoietin receptor, plays a crucial role in the human body's production of platelets. Thrombopoietin, a hormone produced in the liver and kidneys, binds to the TPO receptor, stimulating the production and maturation of megakaryocytes, which are the precursor cells of platelets. This interaction is essential for maintaining adequate platelet levels in the blood, which are vital for normal blood clotting and preventing excessive bleeding. Understanding the TPO receptor's function has led to the development of drugs that target this receptor, offering potential therapeutic options for conditions associated with low platelet counts, such as immune thrombocytopenia and chemotherapy-induced thrombocytopenia.
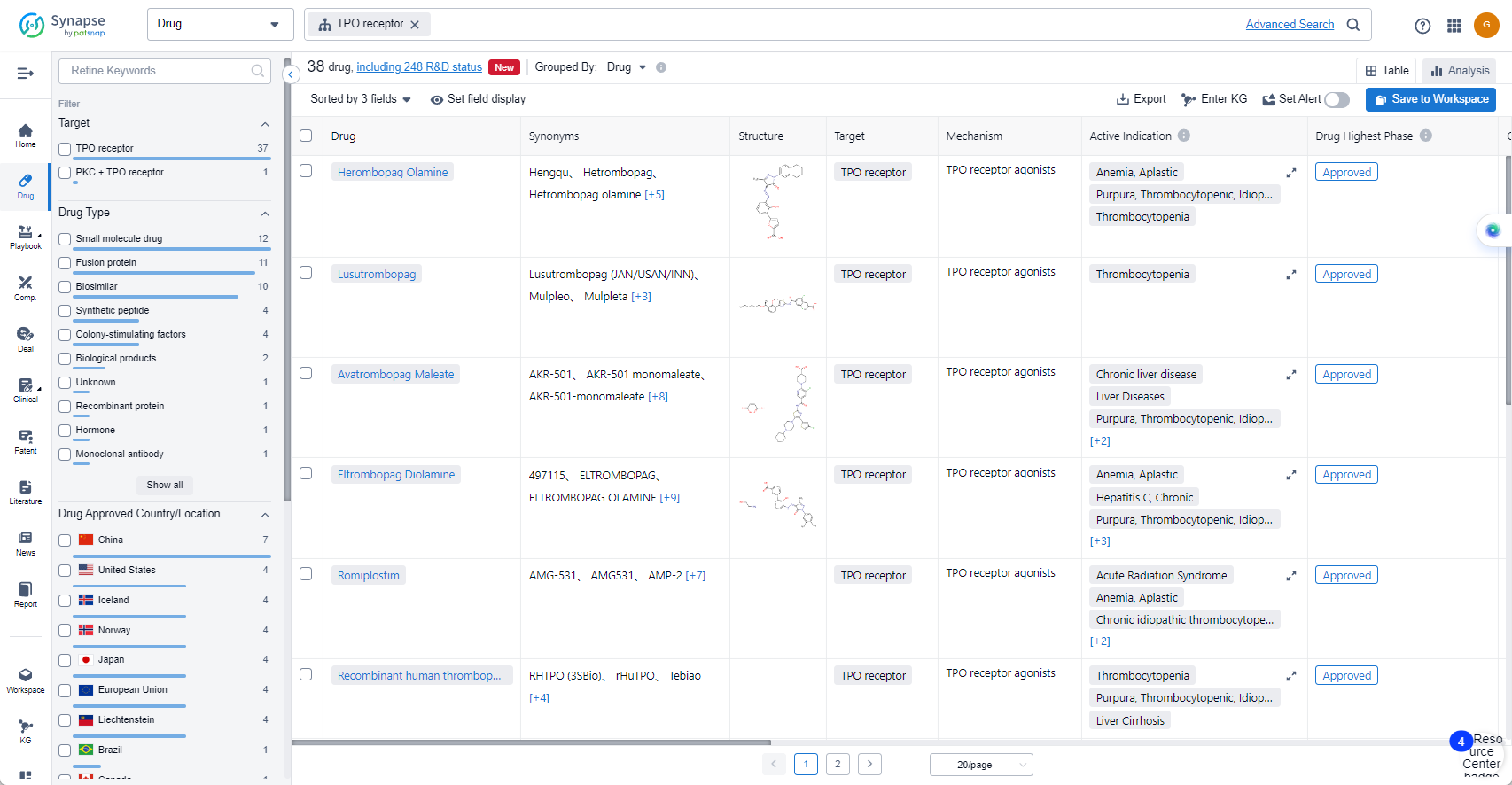
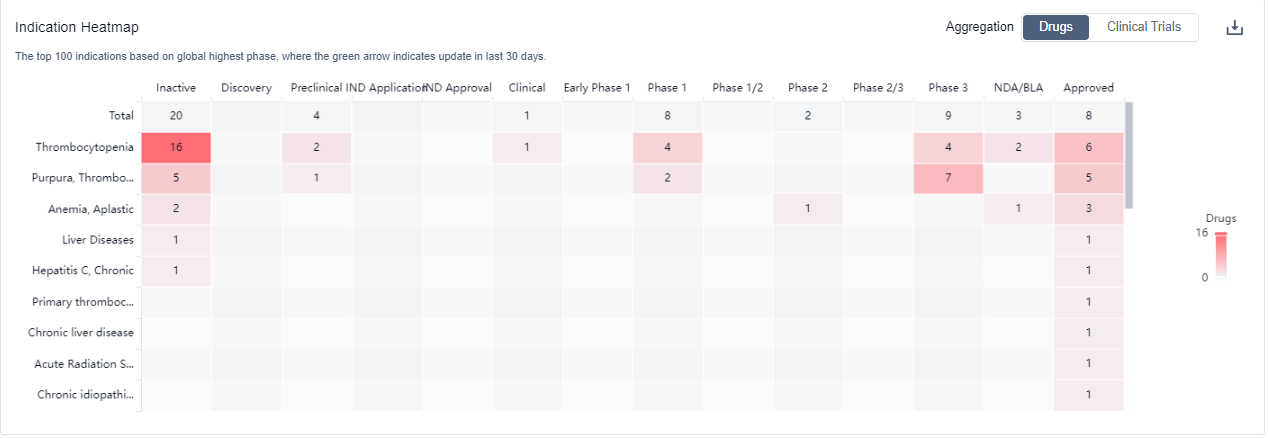
The analysis of the target TPO receptor reveals a competitive landscape with multiple companies actively involved in research and development. The highest stage of development is the "Approved" phase, with several companies having drugs in this phase. Thrombocytopenia and Purpura, Thrombocytopenic, Idiopathic are the most common indications for drugs under this target. Small molecule drugs, Fusion proteins, and Biosimilars are progressing most rapidly, indicating intense competition. China, the United States, the European Union, and Japan are the leading countries in terms of development under this target. Further analysis is required to understand the specific R&D progress of each company, the research institutions involved in biosimilar development, and the factors contributing to the progress in China.
How do they work?
TPO receptor agonists are a type of medication that act on the thrombopoietin (TPO) receptor in the body. Thrombopoietin is a hormone that regulates the production of platelets, which are essential for blood clotting. TPO receptor agonists mimic the action of thrombopoietin and stimulate the production of platelets in the bone marrow.
From a biomedical perspective, TPO receptor agonists are used in the treatment of certain medical conditions that result in low platelet counts, such as immune thrombocytopenia (ITP) and chemotherapy-induced thrombocytopenia. By increasing platelet production, these medications help prevent bleeding episodes and improve overall blood clotting function.
TPO receptor agonists can be administered orally or through injection, depending on the specific drug. They work by binding to the TPO receptor on the surface of bone marrow cells, which triggers a signaling cascade leading to increased platelet production. These medications are typically used when other treatments, such as corticosteroids, have been ineffective or are not suitable for the patient.
It is important to note that TPO receptor agonists may have potential side effects, such as headache, nausea, fatigue, and muscle pain. Close monitoring of platelet counts and regular follow-up with a healthcare professional are necessary during treatment with these medications to ensure optimal effectiveness and safety.
List of TPO receptor Agonists
The currently marketed TPO receptor agonists include:
- Herombopag Olamine
- Lusutrombopag
- Avatrombopag Maleate
- Eltrombopag Diolamine
- Romiplostim
- Recombinant human thrombopoietin(Shenyang Sunshine Pharmaceutical)
- Eltrombopag choline
- NIP-022
- NIP-022
- SCB-219M
For more information, please click on the image below.
What are TPO receptor agonists used for?
TPO receptor agonists are used in the treatment of certain medical conditions that result in low platelet counts, such as immune thrombocytopenia (ITP) and chemotherapy-induced thrombocytopenia. For more information, please click on the image below to log in and search.
How to obtain the latest development progress of TPO receptor agonists?
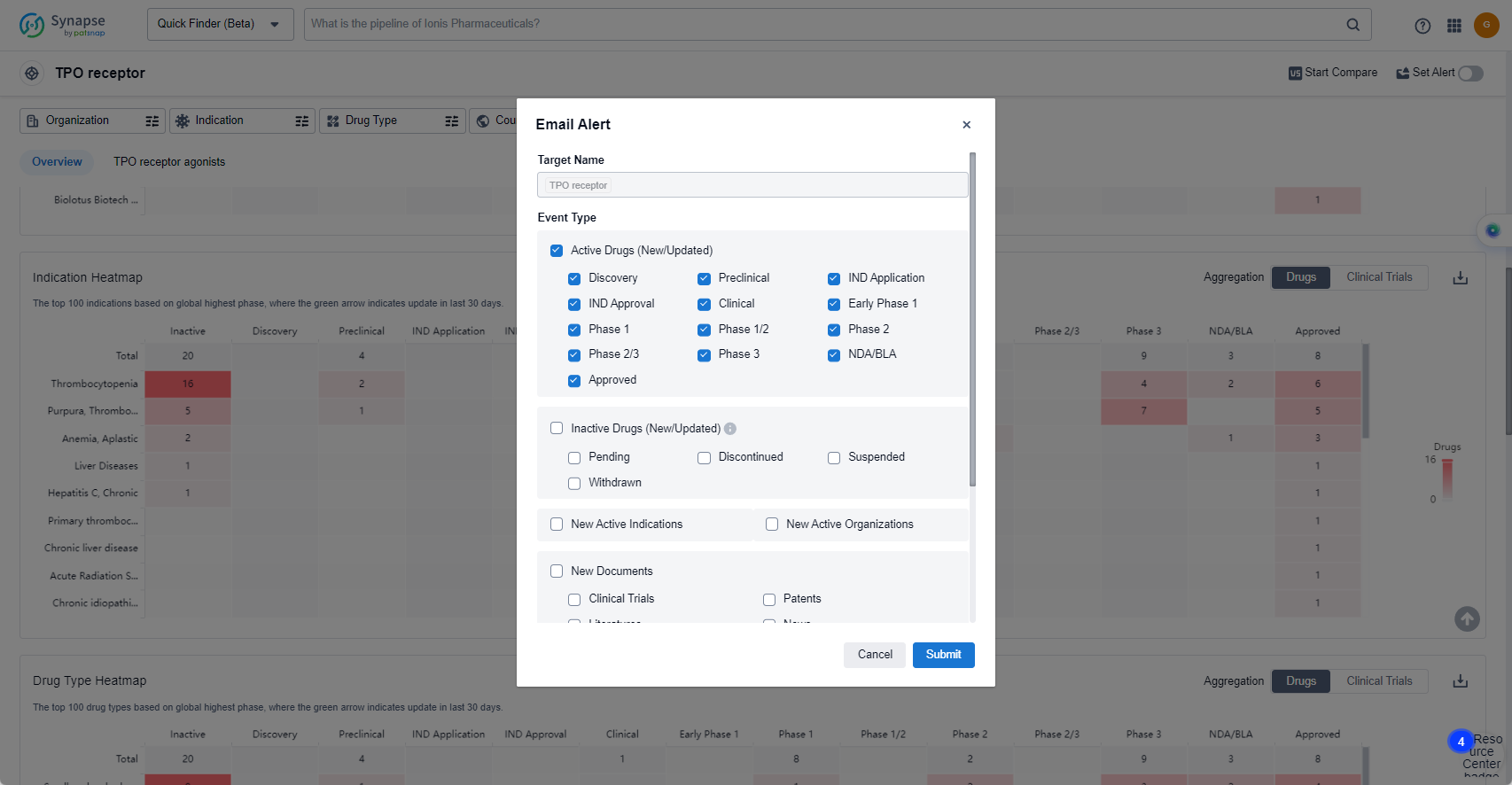
In the Synapse database, you can keep abreast of the latest research and development advances of TPO receptor agonists anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!