Biond Biologics introduces revolutionary anti-ILT3 antibody BND-35, aimed at altering the tumor microenvironment, at 2024 AACR Meeting
Biond Biologics Ltd., a trailblazing biopharmaceutical firm in the clinical phase, known for its groundbreaking work in cancer immunotherapies and a revolutionary system for the internal administration of biologic treatments, is thrilled to share the news that our flagship BND-35 initiative has earned a spot for an in-depth showcase at the prestigious American Association for Cancer Research Annual Meeting. This premier event is set to take place at the San Diego Convention Center, located in San Diego, CA, from the 5th to the 10th of April, 2024.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
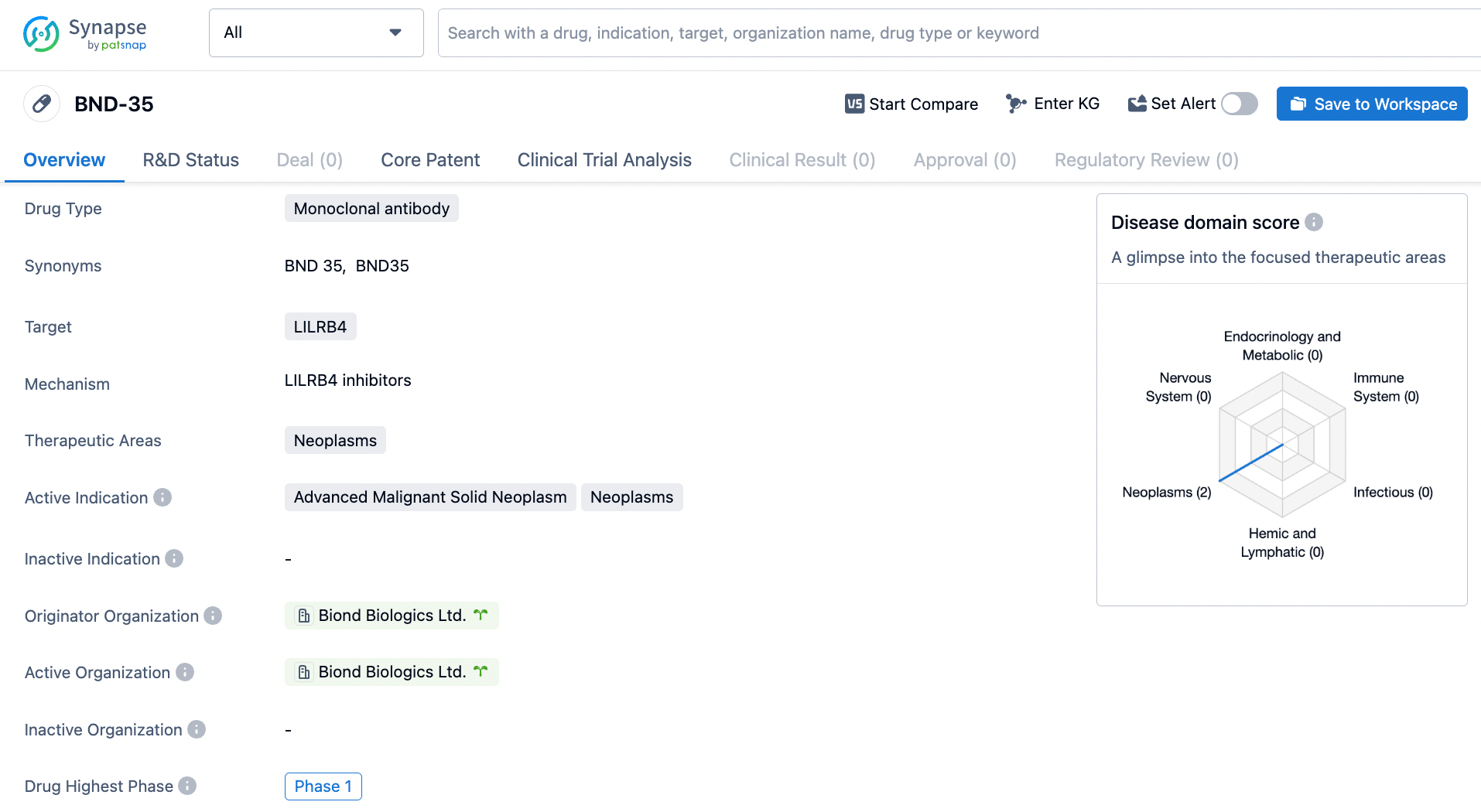
BND-35 emerges as a re-engineered human IgG4, specifically developed as an antagonist to ILT3 (LILRB4). Its primary role is to shift the cancer microenvironment towards an inflammatory state, actively engaging in the suppression of tumor proliferation. The initial phase clinical studies of BND-35 boast a distinctive trial strategy, set to be unveiled at the ACCR event, drawing from Biond’s thorough groundwork and the molecule's proficiency in hampering ILT3's engagement with its diverse ligands.
The forthcoming discourse, titled “Innovative ILT3-Targeting Antibody BND-35: Reshaping the Cancer Microenvironment,” will be featured under the conference theme of “Approaches to Immune Intervention.” Scheduled on Monday, April 8th, 2024, at precisely 4:05 PM, this segment is dedicated to showcasing the extensive groundwork laid by Biond Biologics, emphasizing BND-35’s transformative capability in battling solid tumors.
Biond Biologics' landmark research reveals BND-35’s exclusive capacity to bind to ILT3 with remarkable precision and strength, while preserving the integrity of other receptors in the ILT family. This selective obstruction of ILT3's critical ligand interactions significantly bolsters the inflammatory response in myeloid cells and successfully neutralizes the ILT3-driven suppression of T cells.
The exhibit will communicate insightful findings on BND-35's efficacy,—both as monotherapy as well as when employed in synergy with agents like anti-PD-1 and anti-EGFR. Evidently, the treatment has yielded hopeful signs of reactivating T and NK cell roles, further nurturing an inflammatory Tumor Microenvironment (TME) across a variety of in vitro, ex vivo, and animal study models.
Alongside the presentation spotlighting BND-35, Biond Biologics is actively progressing its Immuno-Oncology portfolio, which includes projects like the pre-clinical BND-67 initiative and the trailblazing INspire approach to intracellular biologic transportation. The discussions will highlight Biond's innovative spirit and dedication to pioneering research and development with the goal of discovering new therapeutic avenues in oncology.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
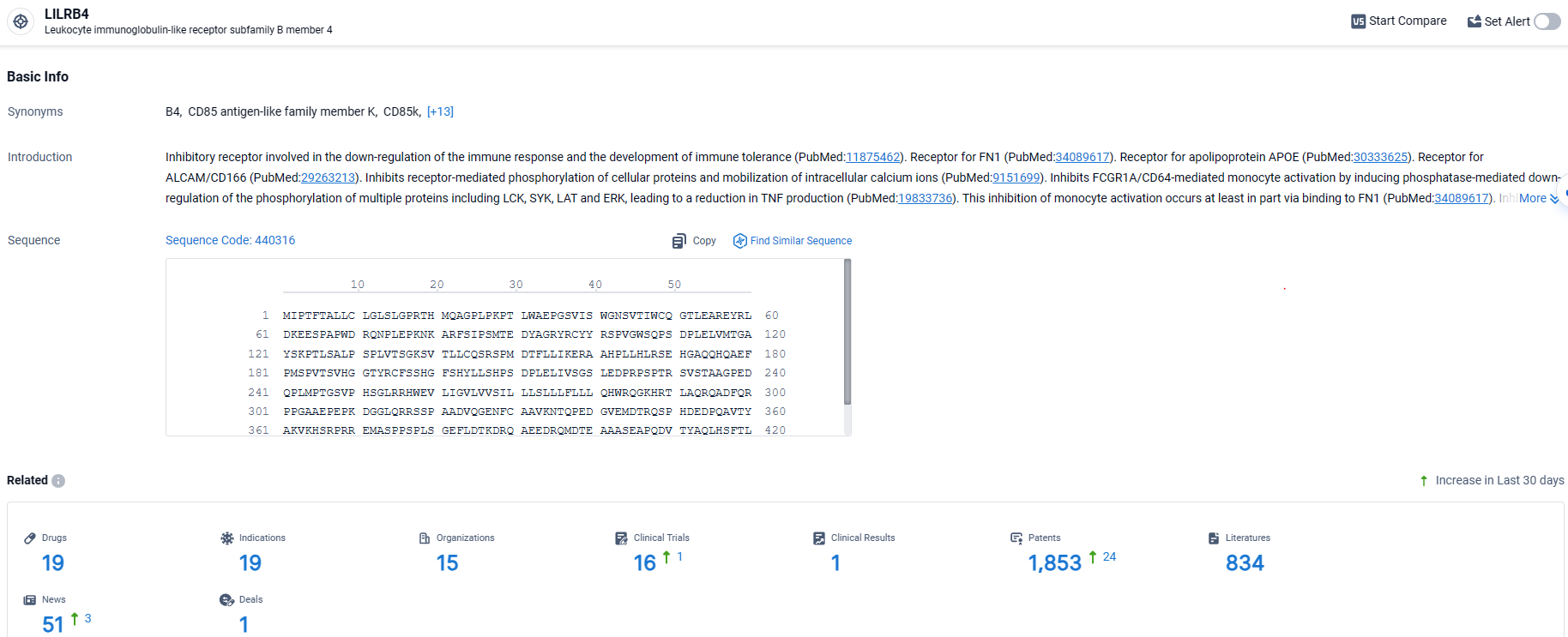
According to the data provided by the Synapse Database, As of April 2, 2024, there are 19 investigational drugs for the LILRB4 target, including 19 indications, 15 R&D institutions involved, with related clinical trials reaching 16, and as many as 1853 patents.
BND-35 is a monoclonal antibody drug developed by Biond Biologics Ltd. It targets LILRB4 and is intended for the treatment of advanced malignant solid neoplasms. The drug is currently in Phase 1 of clinical development, indicating that further research and testing are required to determine its safety and efficacy.