Biotheryx Begins Phase 1 Trial of BTX-9341, a New Dual Action CDK4/6 Degrader, in Monotherapy and Combination Treatments
Biotheryx, Inc., has announced the initiation of dosing for the first patient in its Phase 1 clinical trial of BTX-9341. This investigational oral bifunctional degrader targets cyclin-dependent kinase 4 (CDK4) and cyclin-dependent kinase 6 (CDK6). The trial examines BTX-9341 as both a monotherapy and in combination with fulvestrant for patients with advanced and/or metastatic hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative breast cancer, who have previously undergone CDK4/6 inhibitor therapy in either the adjuvant or metastatic context.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Dr. Leah Fung, CEO of Biotheryx, commented, “BTX-9341 is a strong and highly selective CDK4/6 degrader that has displayed remarkable anti-tumor efficacy in preclinical models, whether they are naïve or resistant to CDK4/6 inhibitors. We are hopeful that this compound will address a crucial unmet medical need for patients with HR+/HER2- breast cancer who have previously undergone CDK4/6 inhibitor treatment. Administering the first dose to a patient in this study marks a significant achievement for Biotheryx, the patients we aim to help, and the scientists who have made this advancement possible.”
The Phase 1 clinical trial will commence with dose escalation of BTX-9341 as a standalone treatment, followed by its combination with fulvestrant. Finally, the trial will include dose expansion of this combination. The study will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of BTX-9341 both as monotherapy and in combination with fulvestrant. After identifying the recommended Phase 2 dose for the combination therapy, a formal efficacy evaluation will be conducted in an expansion cohort.
Dr. Amita Patnaik, Co-Founder and Co-Director of Clinical Research at START, remarked, “BTX-9341 is an exceptionally innovative, first-of-its-kind, potent and selective degrader of CDK4/6, representing a groundbreaking therapeutic strategy. It possesses the potential to revolutionize the treatment landscape for patients with advanced and/or metastatic HR+/HER2- breast cancer, especially for those who have previously been treated with CDK4/6 inhibitors. This milestone is in perfect alignment with START’s mission to hasten drug development and provide early access to state-of-the-art cancer therapies, giving hope to patients and their families.”
In preclinical studies, orally delivered BTX-9341 exhibited effective in vivo degradation of CDK4/6 and enhanced anti-tumor efficacy as a monotherapy compared to standard treatment regimens. Additionally, BTX-9341 showed synergistic effects when combined with selective estrogen receptor degraders like fulvestrant, elacestrant, and camizestrant in both CDK4/6 naïve and resistant models.
BTX-9341 is a first-of-its-kind, oral degrader of CDK4/6, which are important targets for various cancers and have been clinically validated in HR+/HER2- breast cancer. In preclinical breast cancer models, BTX-9341 demonstrated superiority over CDK4/6 inhibitors through its potent and highly selective catalytic degradation of CDK4 and CDK6, robust inhibition of Cyclin E and CDK2 transcription, cell cycle arrest, and ultimately superior efficacy in breast cancer xenografts.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
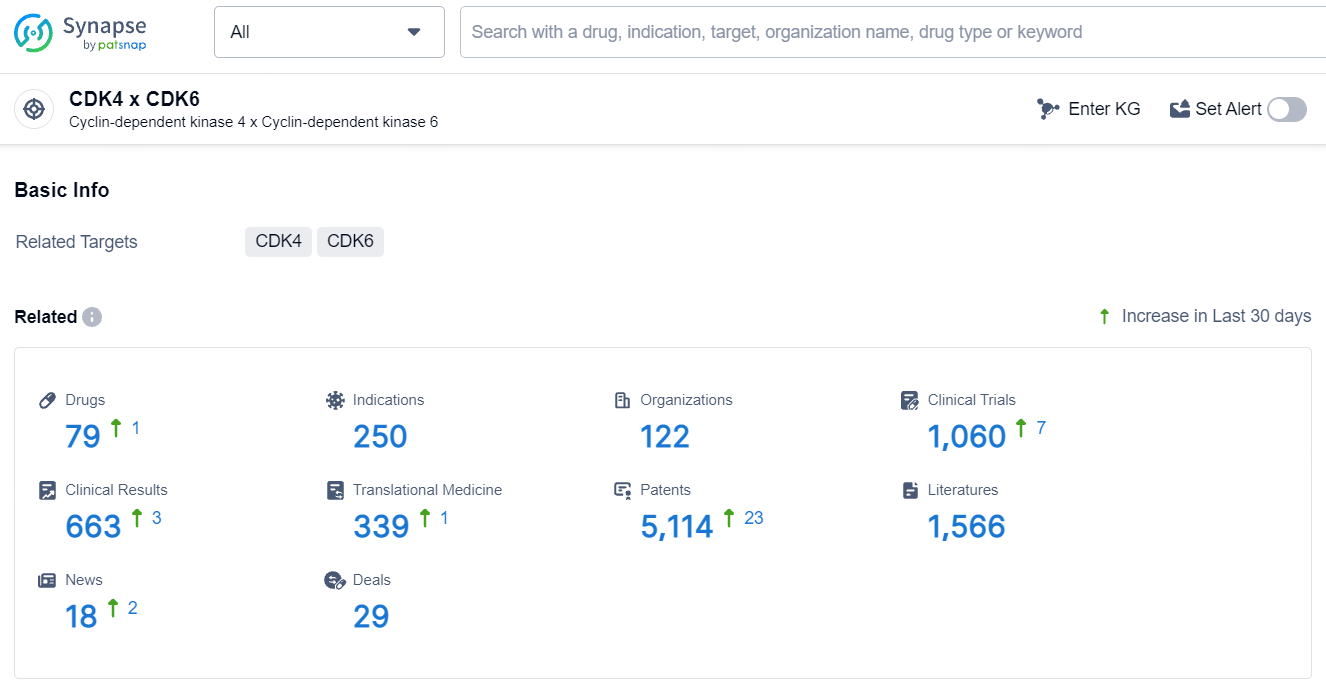
According to the data provided by the Synapse Database, As of July 23, 2024, there are 79 investigational drugs for the CDK4 and CDK6 target, including 250 indications, 122 R&D institutions involved, with related clinical trials reaching 1060, and as many as 5114 patents.
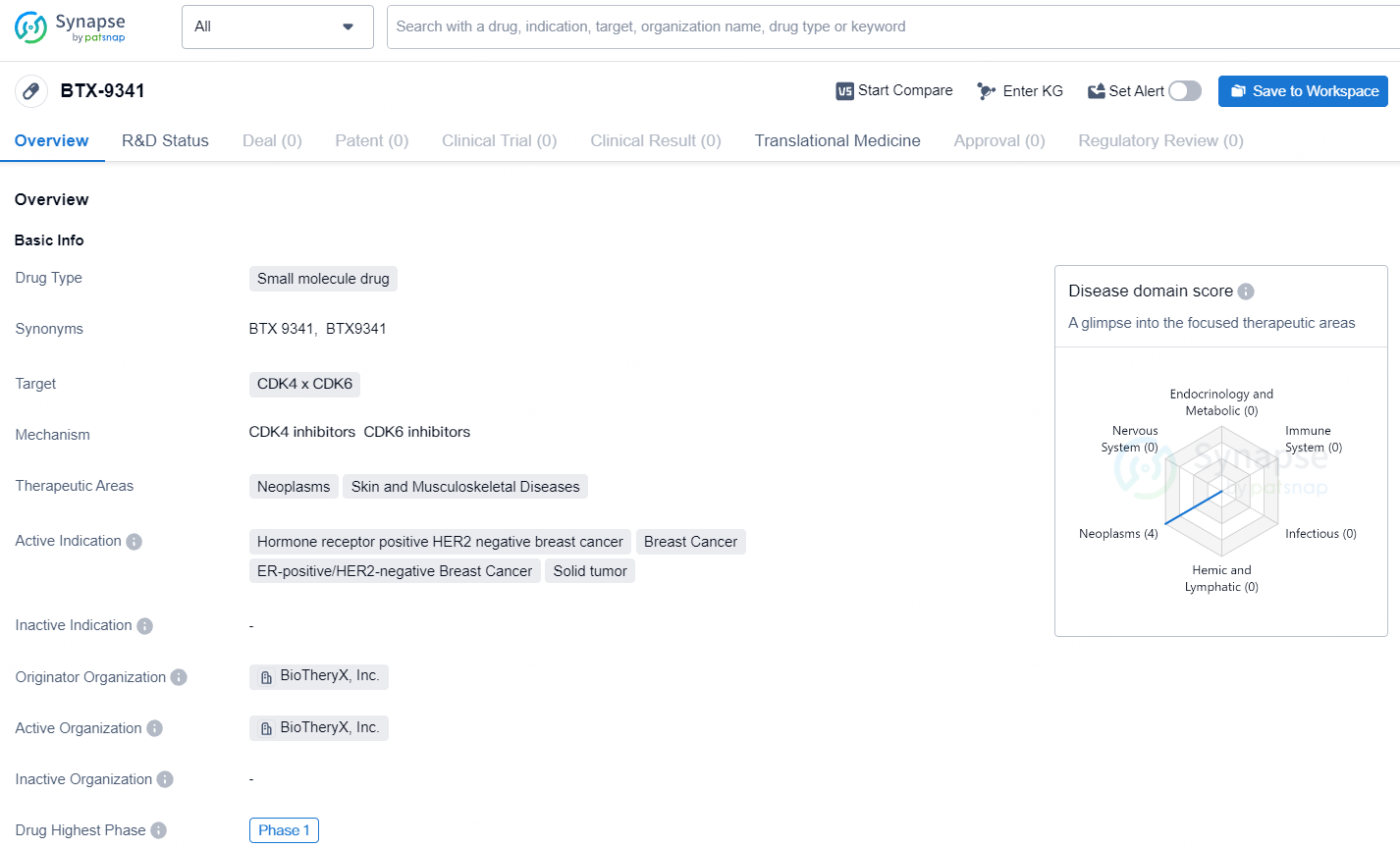
The drug BTX-9341 is a small molecule drug developed by BioTheryX, Inc. The drug targets CDK4 and CDK6, and is intended for the treatment of neoplasms, skin and musculoskeletal diseases. The active indications for BTX-9341 include hormone receptor positive HER2 negative breast cancer, ER-positive/HER2-negative breast cancer, breast cancer, as well as solid tumors.As of the latest available information, BTX-9341 has advanced to Phase 1 of clinical development, making it the highest phase achieved on a global scale. This indicates that the drug has undergone initial testing in humans to evaluate its safety, dosage, and potential efficacy.