Is Oteseconazole approved by the FDA?
Oteseconazole, with the generic name oteseconazole and marketed under the brand name Vivjoa, is an oral capsule formulation primarily used as an azole antifungal medication. It was approved by the US FDA on April 26, 2022. It is approved for the treatment regimen designed to prevent recurrent vulvovaginal candidiasis (RVVC) in women who are not pregnant and not of reproductive potential.
Dosage Form and Drug Class: Oteseconazole is available as an oral capsule with a strength of 150 mg. It belongs to the drug class of azole antifungals, which are commonly used to treat fungal infections by inhibiting the growth of fungi.
What is Oteseconazole Used For? Oteseconazole is specifically used to manage and prevent recurrent vaginal yeast infections (vulvovaginal candidiasis) in women who have a history of such infections. It is not intended for use in females who have not yet had their first menstrual period.
How Does Oteseconazole Work? As an azole antifungal, oteseconazole works by inhibiting the synthesis of ergosterol, a crucial component of fungal cell membranes. By disrupting ergosterol production, it weakens the fungal cell membrane, ultimately leading to cell death and eradication of the infection.
Administration and Dosage: The dosing regimen for oteseconazole varies depending on whether it is used alone or in combination with another antifungal agent like fluconazole. Typically, it involves an initial loading dose followed by maintenance doses to prevent recurrence over a specified period.
Side Effects: Common side effects of oteseconazole include headache and nausea. It is crucial to seek emergency medical help if signs of an allergic reaction occur, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
Warnings and Precautions: Oteseconazole should not be used by pregnant women or those planning to become pregnant, as it may harm an unborn baby. It is also not recommended for use in breastfeeding women. Individuals with known allergies to oteseconazole or similar antifungal medications should avoid its use.
Conclusion: In conclusion, oteseconazole, marketed under the brand name Vivjoa, is FDA approved for the prevention of recurrent vaginal yeast infections in non-pregnant women. It provides a targeted approach to managing this common condition, offering an effective treatment option for those prone to recurrent infections.
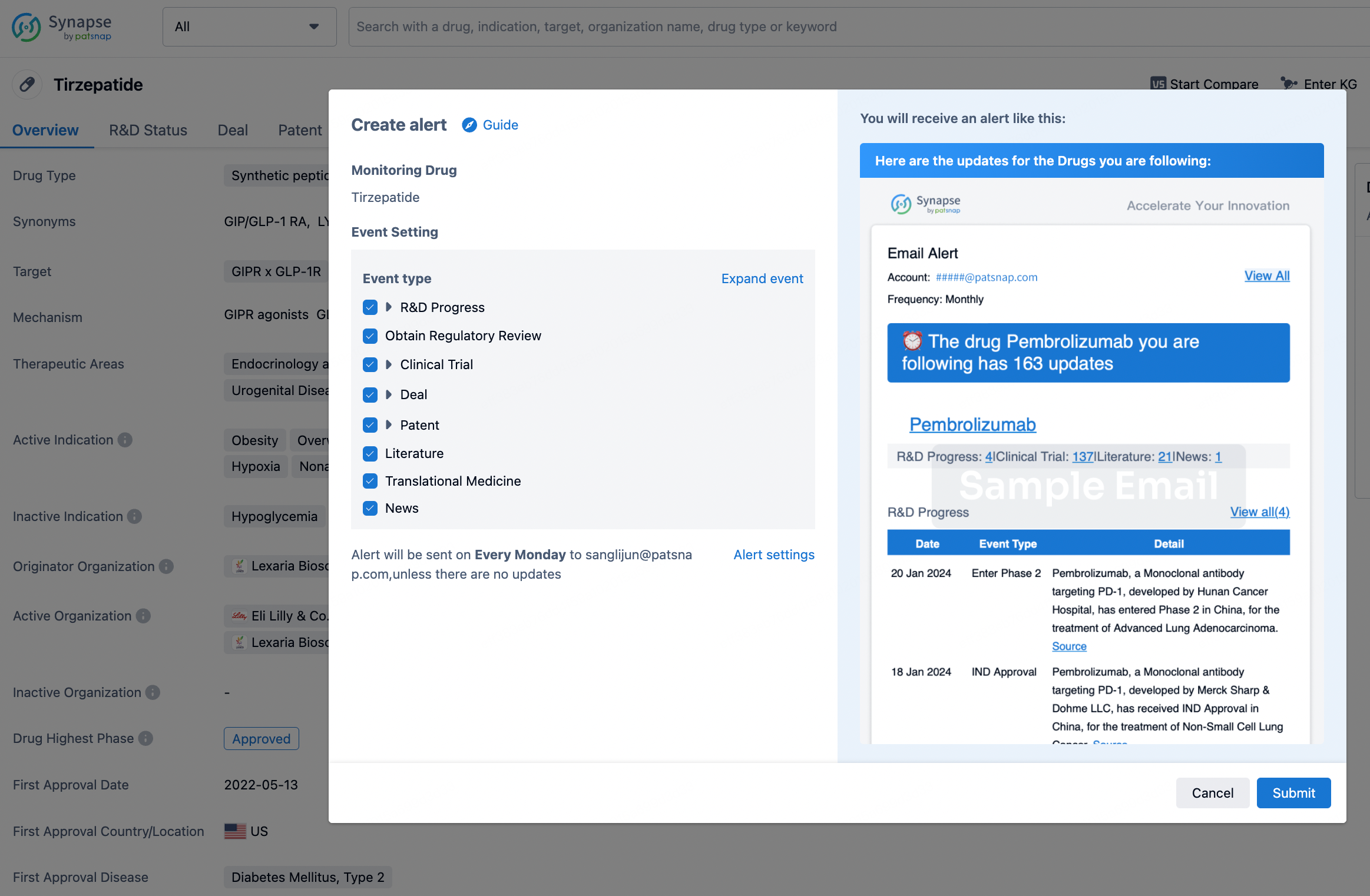
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!