Bolt Biotherapeutics Starts Phase 2 Trial Enrollment for BDC-1001 in HER2+ Breast Cancer Patients Pre-Treated with Enhertu
Bolt Biotherapeutics, Inc., an emerging enterprise at the forefront of crafting innovative immune-centric treatments for oncological conditions, has recently disclosed the initiation of patient dosing in a Phase 2 clinical evaluation. This pivotal study is exploring the efficacy and safety of BDC-1001, a HER2-centric Boltbody™ Immune-Stimulating Antibody Conjugate. The trial is set to assess the therapeutic as both a monotherapy and in a combined regimen with pertuzumab, an antibody also aimed at HER2-positive malignancies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
At the prominent City of Hope cancer research and treatment institution in the United States, the inaugural treatment session was conducted for a patient under the care of Irene Kang, M.D., who heads the Women’s Health Medical Oncology as Medical Director and holds an Assistant Professor position in the Medical Oncology and Therapeutics Research department at City of Hope’s Irvine facility.
Dr. Kang, who is at the helm of the research, remarked on the importance of initiating the dosing with the first participant in the clinical trial. The trial marks an essential step towards discovering more effective therapies for individuals diagnosed with HER2-positive breast cancer that does not respond to existing treatments, despite the availability of several approved HER2-specific drugs for treating advanced stages of the disease. The critical need for alternative therapeutic strategies for patients who experience disease progression is apparent.
Preclinical investigations have indicated that the integration of pertuzumab into treatment regimens can optimize the suppression of tumor growth. The study's focus will be on individuals with HER2-positive metastatic breast cancer, specifically those who have undergone prior therapy with trastuzumab deruxtecan (commercially known as Enhertu®).
Edith A. Perez, M.D., the Chief Medical Officer at Bolt Biotherapeutics, pointed out the limited treatment avenues for patients with HER2-positive breast cancer that have not responded to treatment with Enhertu. She elucidated that BDC-1001 stands out due to its distinctive mode of action that activates the immune response of patients to combat the cancer. The clinical trial represents the first instance to test the impressive anti-cancer properties observed during preclinical studies with BDC-1001 in combination with pertuzumab.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
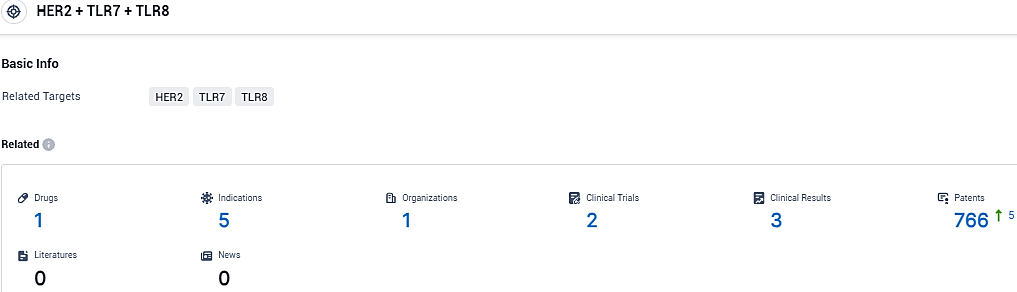
According to the data provided by the Synapse Database, As of December 13, 2023, there are 1 investigational drugs for the HER2 and TLR7 and TLR8 target, including 5 indications, 1 R&D institutions involved, with related clinical trials reaching 2, and as many as 766 patents.
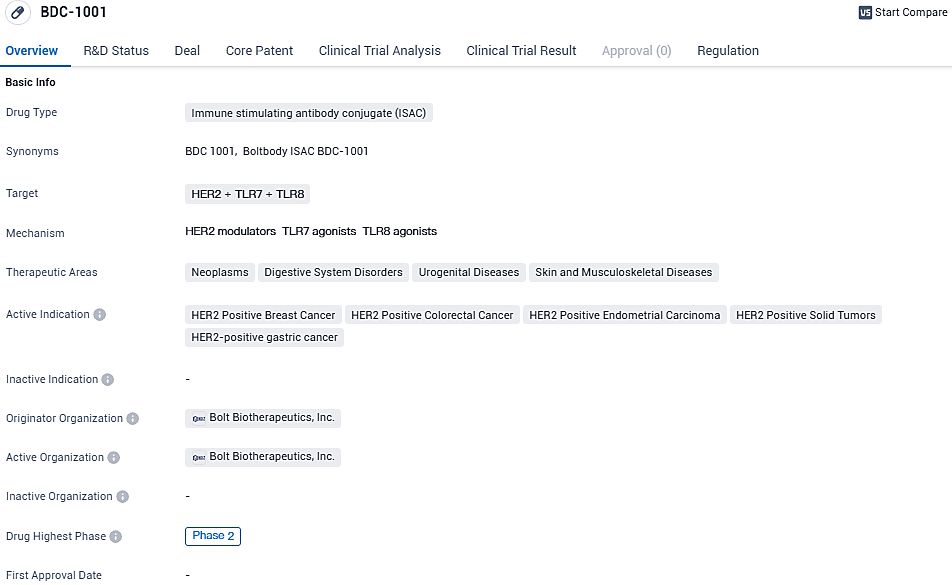
Bolt Biotherapeutics’ lead program, BDC-1001, is a human epidermal growth factor receptor 2 ISAC comprising a HER2-targeting biosimilar of trastuzumab conjugated with a non-cleavable linker to a proprietary TLR7/8 agonist. Following the successful completion of the BDC-1001 dose-escalation trial for the treatment of patients with HER2-expressing solid tumors, Bolt is now conducting two Phase 2 clinical trials in the U.S., Europe, and South Korea for patients with colorectal, endometrial, and gastroesophageal cancers,breast cancer.