Coherus Presents Casdozokitug Early Results at ESMO 2023 Immuno-Oncology
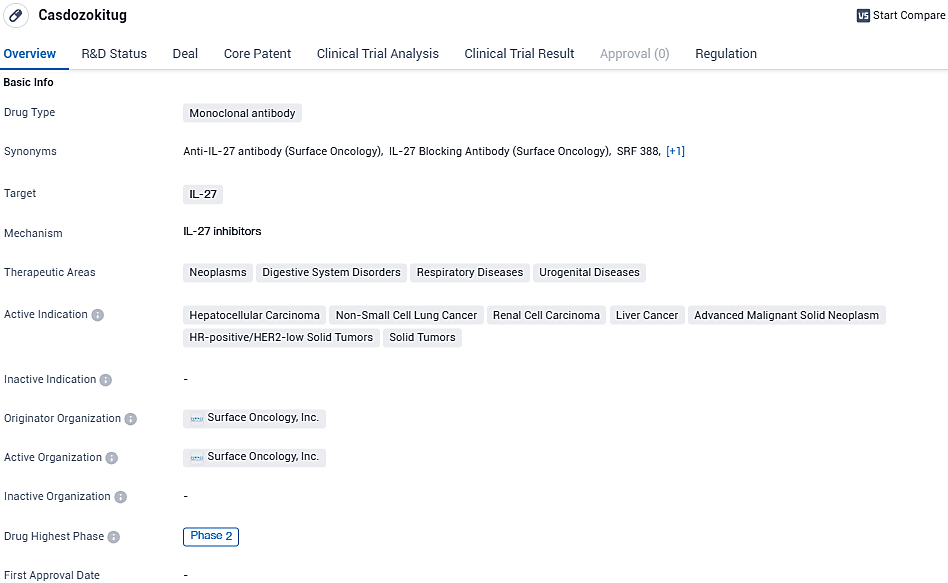
Coherus BioSciences, Inc. has unveiled results from its active Phase 1b/2 study involving the innovative IL-27-targeting antibody, casdozokitug (casdozo). This information is being shared at the ESMO Immuno-Oncology Congress for the year 2023, which is scheduled from the 6th to the 8th of December at the Palexpo Exhibition Centre, located in Geneva, Switzerland.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This briefing consists of the most recent figures on individual dosage increases and group expansions for casdozo use alone, as well as an analysis of the effects from combining casdozo with pembrolizumab, an anti-PD-1 antibody, on subjects with advanced non-small cell lung cancer (NSCLC) who have not responded to 2-4 prior treatment regimes, which includes chemotherapy and anti-PD-1 therapy.
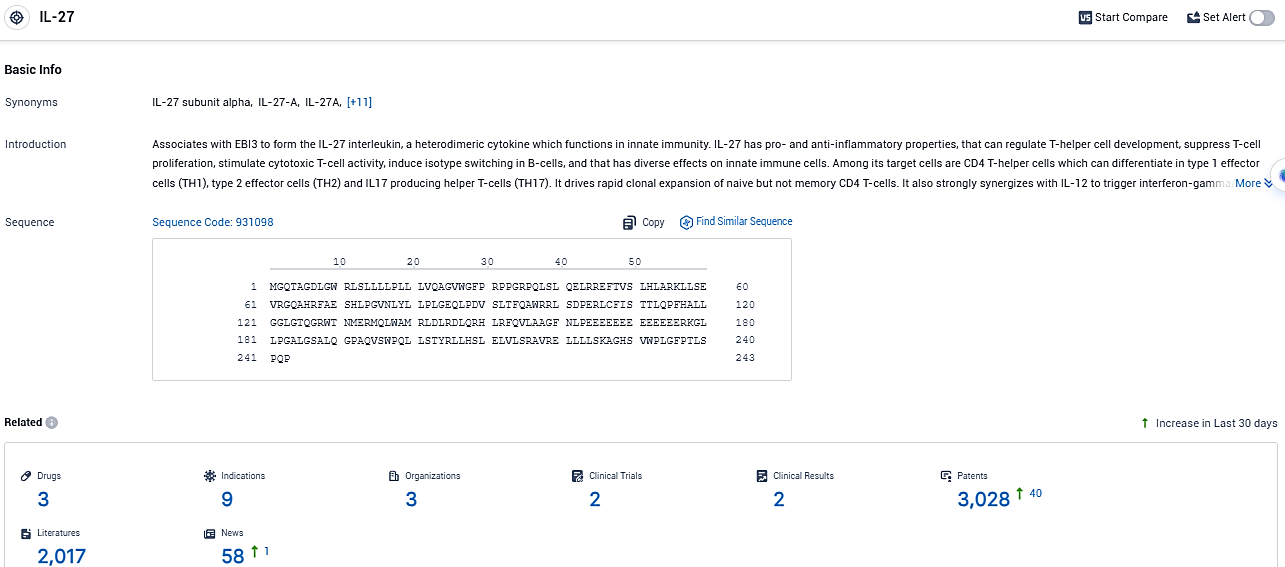
Interleukin (IL)-27 plays a central role as an immunoregulatory cytokine that hampers immune system's abilities to combat tumors, making it a significant new focus for oncological medical treatments. Representing a unique entity, casdozo stands as the sole antibody of its class at the clinical testing phase that antagonizes the cytokine IL-27.
"Tumors described as 'cold' or 'excluded' within the immune microenvironment often pose significant resistance to checkpoint blockade therapies, offering a distinctive challenge within the realm of immuno-oncology," stated Thomas Marron, M.D., Ph.D., who serves as the Director for the Early Phase Trials Unit at The Tisch Cancer Center. He further noted, "The production of IL-27 by macrophages within the tumor could be a vital process that helps the tumor to deter critical anti-tumor lymphocytes from attacking it."
"In patients who have previously experienced treatment failure with PD-1 inhibitors, the results have been quite promising, and they strongly support further research, both as subsequent options and as possible initial therapy. We are quite fascinated by the outcomes in those patients affected by squamous cell carcinoma. By analyzing both tissue and blood samples from these individuals, our goal is to discover predictive biomarkers that may help us better identify who is more likely to benefit from treatments such as casdozo," added Marron.
"We are gratified by the evidence showing that casdozo not only prompts immune response but also displays standalone abilities to shrink tumors while maintaining an acceptable safety record for NSCLC patients. These findings reinforce the blockade of IL-27 as an innovative approach for disrupting immune resistance that many patients with stubborn solid tumors encounter," commented Rosh Dias, the Chief Medical Officer at Coherus.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 12, 2023, there are 3 investigational drugs for the IL-27 target, including 9 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 3028 patents.
Casdozokitug is a first-in-class human anti-IL-27 antibody designed to inhibit the activity of this immunosuppressive cytokine. Casdozokitug has been granted Orphan Drug designation and Fast Track designation for the treatment of refractory hepatocellular carcinoma from the FDA. It is the first IL-27 antibody to enter the clinic.