Bristol Myers Squibb Invests in Cancer Immunotherapy Targeting GPR65
On April 18th, Pathios Therapeutics, a biotech company dedicated to developing pioneering new cancer treatments, announced that it had raised $25 million in the initial tranche of its Series B financing. This round of funding includes new strategic investment from Bristol Myers Squibb, as well as support from existing investors Canaan and Brandon Capital. The funds raised will support the development of Pathios's unique cancer immunotherapy, which involves developing small molecule antagonists to inhibit the activity of the GPR65 receptor, a novel target genetically associated with a variety of immune-mediated diseases. The company expects to advance its internally discovered oral, potent, selective small molecule GPR65 inhibitor, PTT-4256, into clinical trials for advanced solid tumors by the end of 2024.

Pathios, founded in 2017, is a drug discovery and development company dedicated to transforming innovative science into novel therapeutics. The company is focused on developing small molecule inhibitors targeting pH-sensing G protein-coupled receptor GPR65 to combat the immune suppressive polarization of immune cells, including tumor-associated macrophages, triggered by the acidic tumor microenvironment prevalent in most cancers.
GPR65 and tumor
The tumor microenvironment plays a crucial regulatory role in the initiation and progression of cancer. Despite the emergence of T-cell checkpoint inhibitors sparking a revolution in the treatment of various types of cancer, the response of most patients to these therapies remains less than ideal. The fundamental reason for this suboptimal response lies in the key characteristics of the immunologically hostile tumor microenvironment present in many solid tumors. These characteristics include high vascularization and wound healing gene signatures, reduced expression of pro-inflammatory cytokines and chemokines, suppressed interferon and antigen-presenting genes, along with the dominance of immunosuppressive tumor-associated macrophages (TAMs) and other protumorigenic myeloid cells.
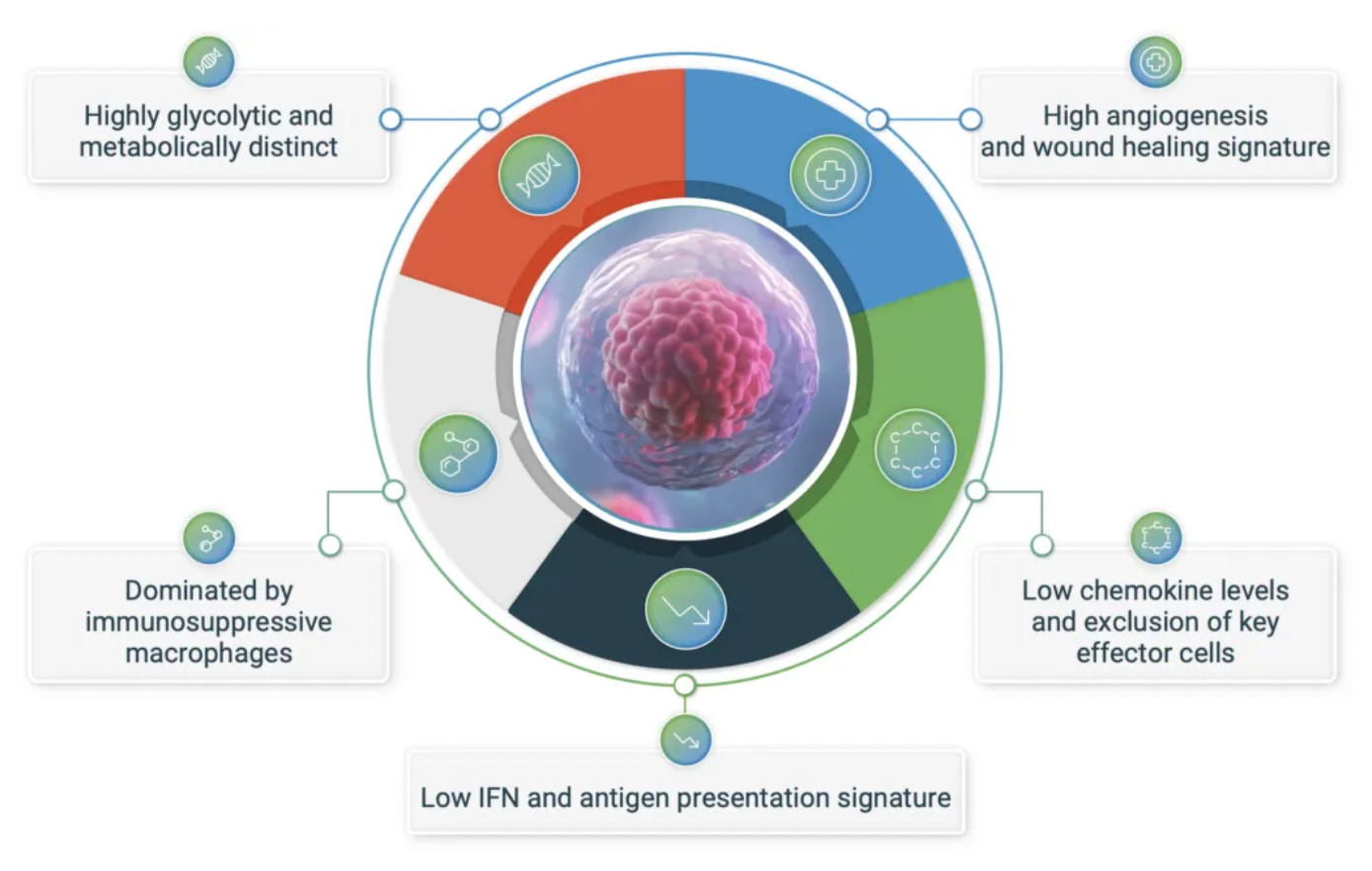
The GPR65-related signaling pathway plays a crucial role in regulating the tumor microenvironment. In many cancers, the tumor microenvironment tends to become acidic due to the well-known metabolic shift from oxidative phosphorylation to anaerobic glycolysis, coupled with high expression of acidic enzymes. At the local TME level, a low pH value signals through GPR65 on a variety of immune cells, leading to a shift in their characteristics towards a pro-tumor phenotype.
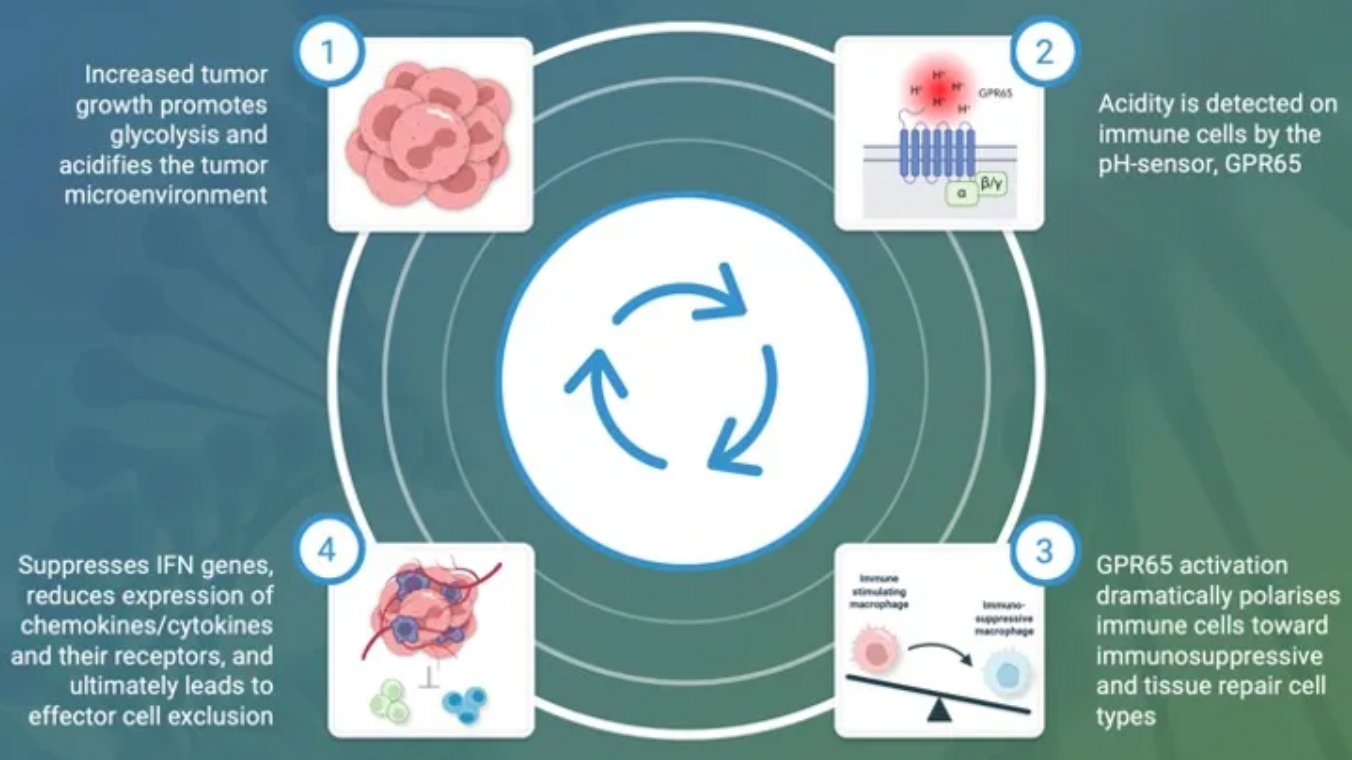
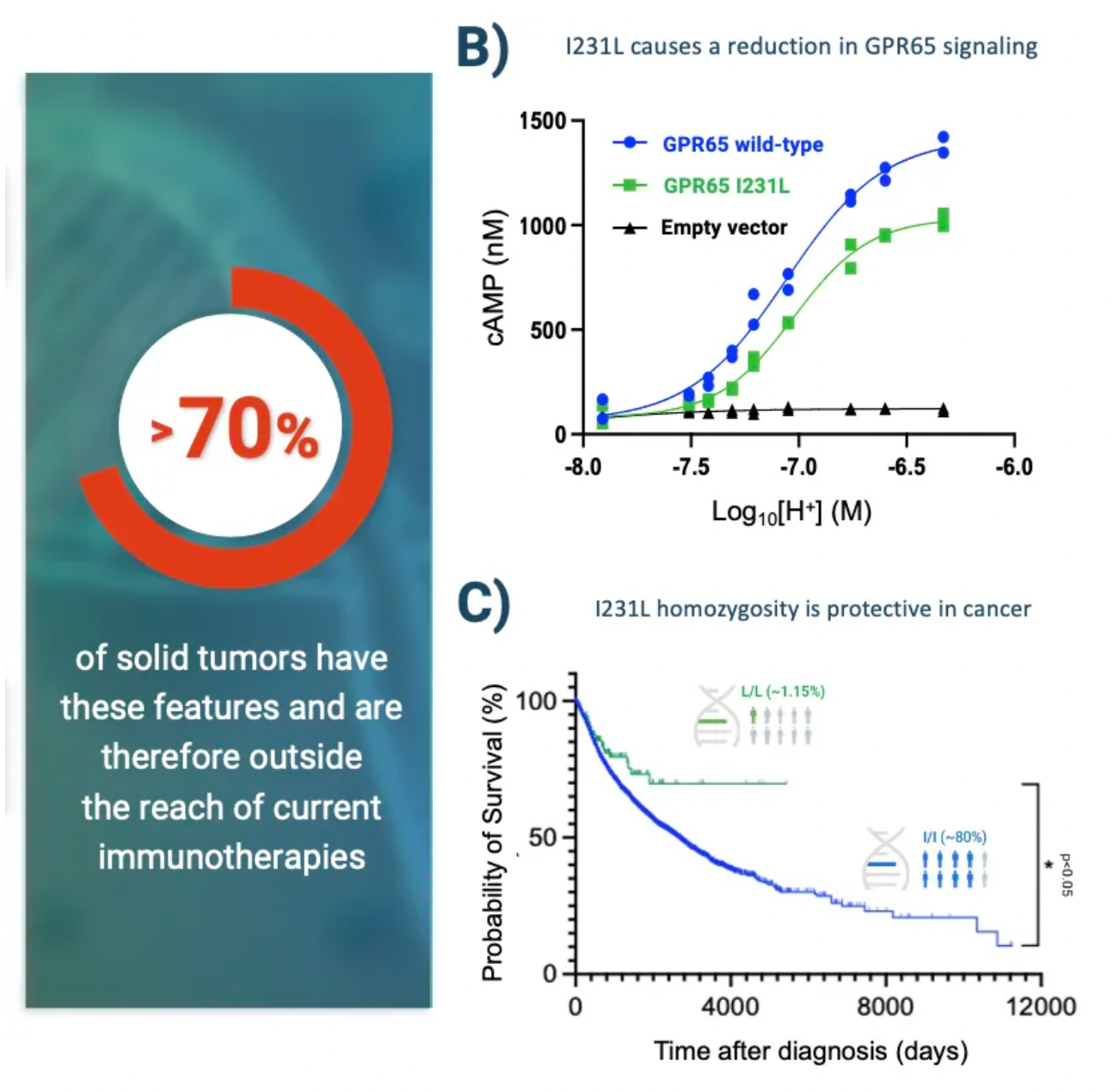
By examining the effects of coding variations in the human GPR65 gene on the inhibition of the GPR65 signaling pathway, early studies suggest that this pathway has potential in cancer therapy.
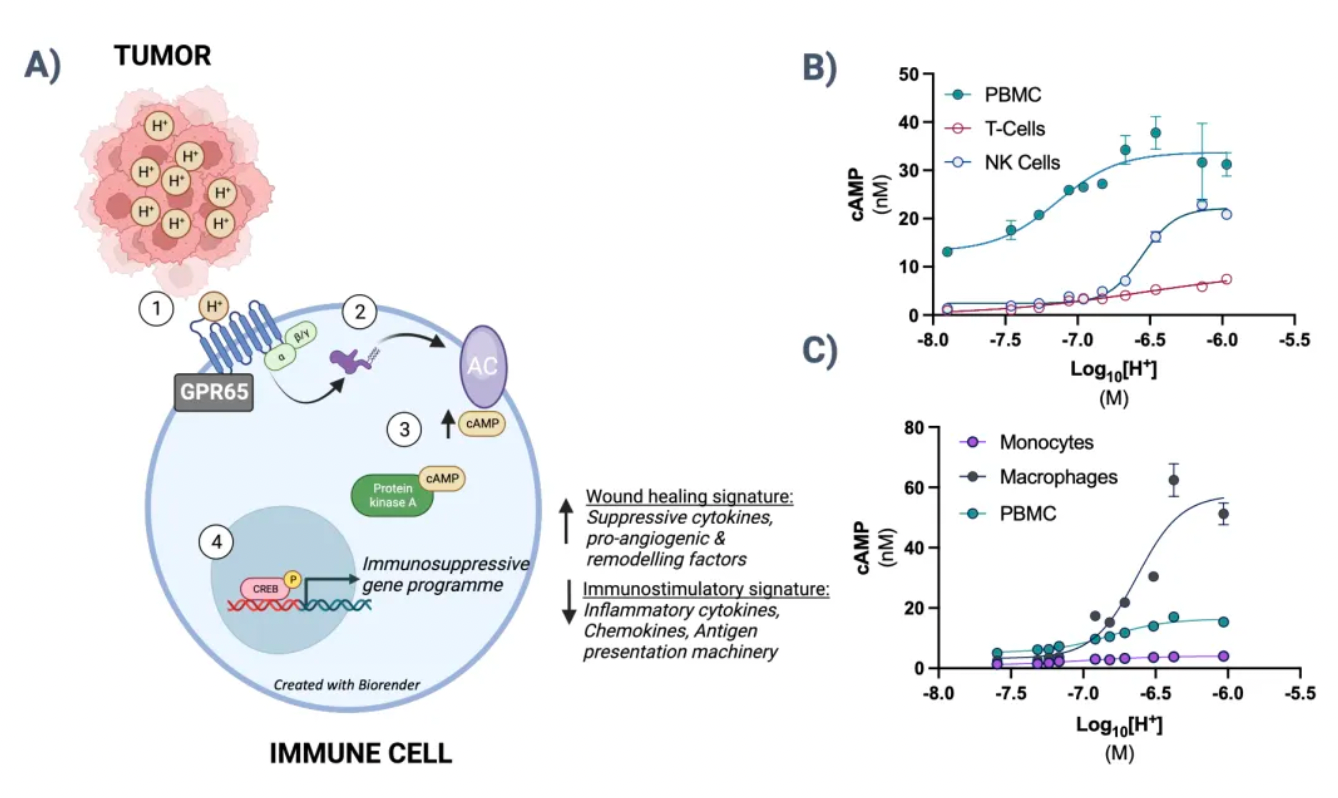
The variant (I231L) of the GPR65 gene results in reduced receptor signaling, and patients who are homozygous for this coding change (representing about 1.5% of all cancer patients) show a significant survival advantage compared to patients with other genotypes. This survival advantage persists even in patients with tumors that are highly glycolytic and expected to respond poorly to current T-cell checkpoint immunotherapies. Furthermore, extensive preliminary pharmacological work conducted in a series of syngeneic mouse models indicates that inhibiting GPR65 has the potential to achieve profound monotherapy activity.
Pathios is exploring a novel immunooncological approach to treat cancer, aiming to combat the immunosuppressive polarization of immune cells (including tumor-associated macrophages, TAMs) triggered by the acidic tumor microenvironment. This goal is achieved by targeting GPR65, an acid-sensing G protein-coupled receptor that has been proven to promote immunosuppressive polarization of immune cells, attacking a common pathway for cancer immune evasion.
In 2019, Pathios announced a strategic partnership with Sygnature Discovery to accelerate Pathios' drug discovery and development initiatives. Under the terms of the agreement, Pathios and Sygnature will collaborate on a comprehensive drug discovery project targeting GPR65. Sygnature will apply its industry-leading expertise in the field of GPCR (G protein-coupled receptors) bioscience and medicinal chemistry to enhance Pathios' ongoing lead compound optimization program. Furthermore, Sygnature will use its capabilities in computational chemistry, library design and synthesis, and high-throughput screening to broaden the scope of Pathios' hit compound discovery. As part of the agreement, in addition to financial compensation, Sygnature will also receive equity.

The preclinical data presented on PTT-4256 at the 2023 American Association for Cancer Research (AACR) Annual Meeting demonstrated that the compound exhibits excellent drug-like properties and impressive single-agent antitumor activity. Importantly, PTT-4256 has been proven to be highly effective against the induction of macrophage polarization towards an immunosuppressive state in low pH conditions (i.e., high acidity) both in humans and mice. This immunosuppression alleviation induced by PTT-4256 is associated with a dose-dependent increase in a spectrum of pro-inflammatory genes consistent with antitumor immune responses, indicating the infiltration of T cells and natural killer (NK) cells into the Tumor Microenvironment (TME), and the prevention of immunosuppressive cytokines activation.