Caliway Announces FDA Phase II Trial Approval for Dercum's Syndrome Candidate CBL-514
Caliway Biopharmaceuticals has revealed the U.S. FDA's acknowledgment of their submission for an Investigational New Drug (IND), code-named CBL-514, for the CBL-0202 DD-dedicated Phase 2 clinical trial aimed at addressing Dercum's Disease.
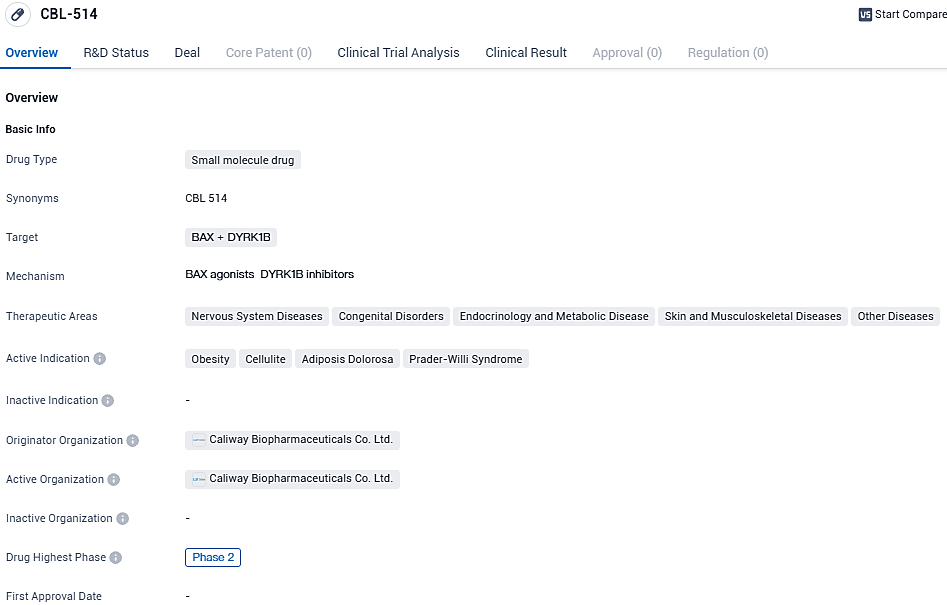
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dercum's disease is an uncommon medical condition marked by the development of painful fat tumors known as lipomas. These growths can cause significant, enduring pain (lasting more than 3 months), which frequently hinders a patient's abilities. To date, medical organizations have not sanctioned any specific therapeutic regimen for Dercum's disease, which has triggered a surge in the worldwide market for treatment, reaching an estimated value of $22.75 billion by 2032.
Initial results from the CBL-514 Phase 2 trial reported successful outcomes, with lipoma size diminishing by upwards of 50% and a notable decrease in pain levels by 4.7 points on the relevant scale. This promising therapy stands on the brink of possibly becoming the inaugural officially sanctioned medication for managing Dercum's disease.
The upcoming CBL-0202DD Phase 2 trial is set to probe deeper into the effectiveness and safety profile of CBL-514, alongside its tolerance compared to a placebo control. The trial is slated to begin patient enrollment in the second quarter of 2024.
Characteristics of Dercum's disease include the emergence of painful lipomas predominantly situated on the torso and nearby limbs. The associated pain is symmetrical, persistent (lasting beyond three months), typically impairs normal function, and does not respond well to standard pain relievers. An early examination of the disease's manifestations highlighted a trio of principal concerns: excessive weight gain, discomfort due to lipomas, and a spectrum of psychological responses such as disrupted sleep, depressive episodes, and heightened anxiety.
The origins of Dercum's disease are yet to be unraveled. Therapies currently on offer, encompassing surgical excision, liposuction, electrotherapeutic methods, and use of anti-inflammatory drugs, provide only symptomatic respite and come with a high risk of undesirable consequences. Therefore, the demand for an effective clinical approach to Dercum's disease remains a significant gap in healthcare services.
According to the Global Dercum's Disease Market Research Report, the treatment market for this condition stood at $11.3 billion as of 2021. A forecasted compound annual growth rate of 6.76% suggests that, by the year 2030, this figure will likely balloon to approximately $19.95 billion.
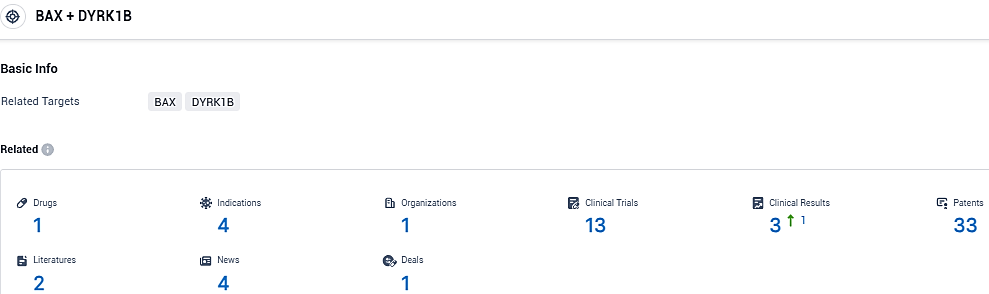
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 12, 2024, there are 1 investigational drugs for the BAX and DYRK1B target, including 4 indications, 1 R&D institutions involved, with related clinical trials reaching 13, and as many as 33 patents.
CBL-514 appears to be a promising small molecule drug with a diverse range of potential therapeutic applications. Its targeting of BAX and DYRK1B, along with its active indications in various diseases, suggests that it may have a broad impact in the field of biomedicine. However, further research and clinical trials are needed to fully assess its safety and efficacy before it can be considered for commercialization.