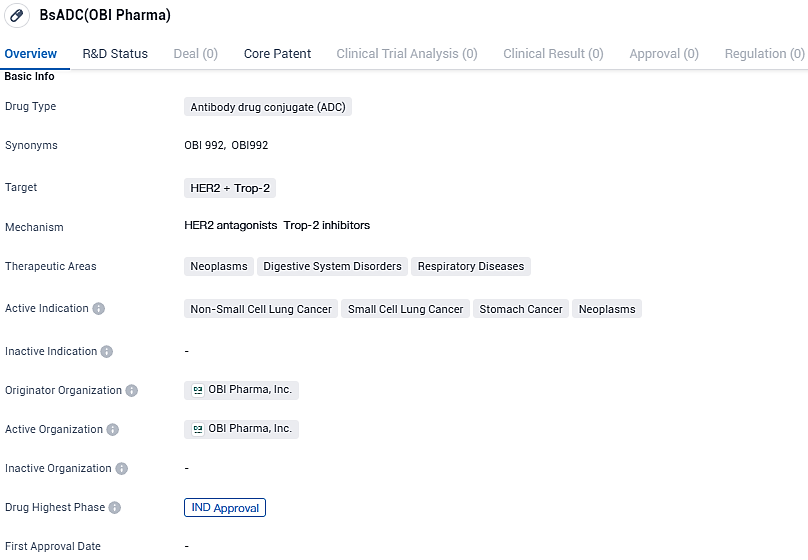
OBI Pharma announced FDA approval for a Phase 1/2 trial of its TROP2 ADC, OBI-992
OBI Pharma, a collaborative affiliate of Biosion Inc., has revealed the U.S. FDA's approval for the exploration of a new therapeutic agent, OBI-992, allowing the initiation of a Phase 1/2 clinical trial to evaluate the innovative antibody-drug conjugate aimed at treating cancer through the TROP2 target.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The specialized antibody in question was identified utilizing the high-efficiency SynTracer® High Throughput Endocytosis Platform developed by Biosion and subsequently secured under a licensing agreement by OBI Pharma in December 2021. While OBI Pharma maintains the rights to market OBI-992 outside of China, Biosion retains the rights within the Chinese market.
Hugh M. Davis, Ph.D., who serves as the Chief Business & Development Officer and President of Biosion USA, Inc., expressed his contentment with the FDA's approval for OBI-992. "The preclinical trials have shown that OBI-992 surpasses competing TROP2 ADCs," he observed. "This confirms the superior capability of Biosion's SynTracer® platform in selecting the most suitable antibodies for advancing novel ADC treatments."
The Chief Medical Officer of OBI Pharma, Wayne Saville, M.D., remarked on the anticipated clinical assessment, stating: "Our goal with this clinical study is to explore OBI-992's safety profile, its pharmacokinetic properties, and its initial effectiveness. This novel TROP2-targeted ADC holds the potential to lead its class. The commencement of patient administration in the Phase 1/2 study for OBI-992 is slated for early 2024, and we eagerly await this milestone."
Heidi Wang, Ph.D., the Chief Executive Officer at OBI Pharma, contributed her insights, saying, "Our team at OBI Pharma crafted the novel anti-TROP2 ADC known as OBI-992. Its excellence in preclinical performance is marked by impressive efficacy, a strong safety profile, and remarkable in-vivo stability when compared with other TROP2-targeting ADCs. We are thrilled to initiate the first-in-human clinical trial for OBI-992, as we aim to bring our innovative cancer treatments from the laboratory to patient care."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
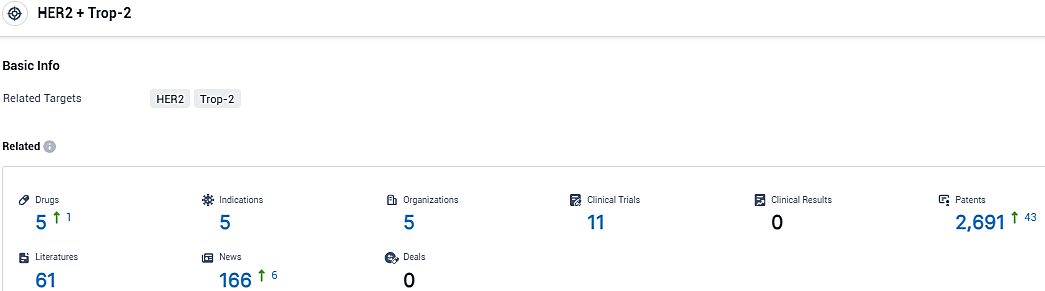
According to the data provided by the Synapse Database, As of January 12, 2024, there are 5 investigational drugs for the HER2 and Trop-2 target, including 5 indications, 5 R&D institutions involved, with related clinical trials reaching 11, and as many as 2691 patents.
OBI-992 demonstrates remarkable antitumor efficacy, improved pharmacokinetic characteristics, and a favorable safety profile in animal models. The anti-TROP-2 targeting antibody was discovered and developed by Biosion, OBI Pharma owns ex-China commercial rights for OBI-992.