Candel Therapeutics' CAN-2409 Achieves Phase 3 Success in Prostate Cancer
Candel Therapeutics, Inc. (Nasdaq: CADL), a biopharmaceutical firm in the clinical development phase that specializes in creating multimodal biological immunotherapies to assist patients in battling cancer, has announced the findings from a multicenter phase 3 clinical trial assessing the efficacy of CAN-2409 viral immunotherapy in patients with localized prostate cancer.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the U.S. alone, more than 100,000 men receive a diagnosis of localized prostate cancer each year, and over 50,000 are currently undergoing radiotherapy. Prostate cancer remains the second most common cause of cancer mortality among American men, and for more than two decades, there have been no new treatments or noteworthy changes to the standard care protocol for localized, non-metastatic prostate cancer. The market for localized prostate cancer therapies, specifically for CAN-2409, has the potential to exceed $10 billion within the United States.
The phase 3 clinical trial for CAN-2409 in patients with intermediate-to-high-risk, localized prostate cancer achieved its primary goal by showing a statistically significant enhancement in disease-free survival (DFS) for those treated with CAN-2409 in conjunction with the prodrug valacyclovir and standard care, compared to standard care alone.
This 2:1 randomized, double-blind, placebo-controlled, multicenter study included 745 participants (intention-to-treat population, ITT) and aimed to assess both the efficacy and safety of CAN-2409 combined with valacyclovir viral immunotherapy alongside conventional external beam radiation therapy to enhance DFS in intermediate-to-high-risk localized prostate cancer patients. Participants were randomized and stratified based on the administration of short-term androgen deprivation therapy (ADT) lasting less than six months.
CAN-2409 is an experimental, off-the-shelf adenovirus that is replication-defective and delivers the herpes simplex virus thymidine kinase (HSV-tk) gene specifically to tumor cells. When used in conjunction with valacyclovir, CAN-2409 aims to trigger immunogenic cell death in the tumor cells while unveiling tumor antigens within an activated tumor microenvironment. This therapeutic approach seeks to promote a tailored and targeted response from CD8+ T cells against the tumor based on the in situ vaccination of diverse tumor antigens. Both preclinical and clinical studies have indicated that CAN-2409 may work synergistically with local radiotherapy, reinforcing the rationale for the design of the ongoing phase 3 clinical trial.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
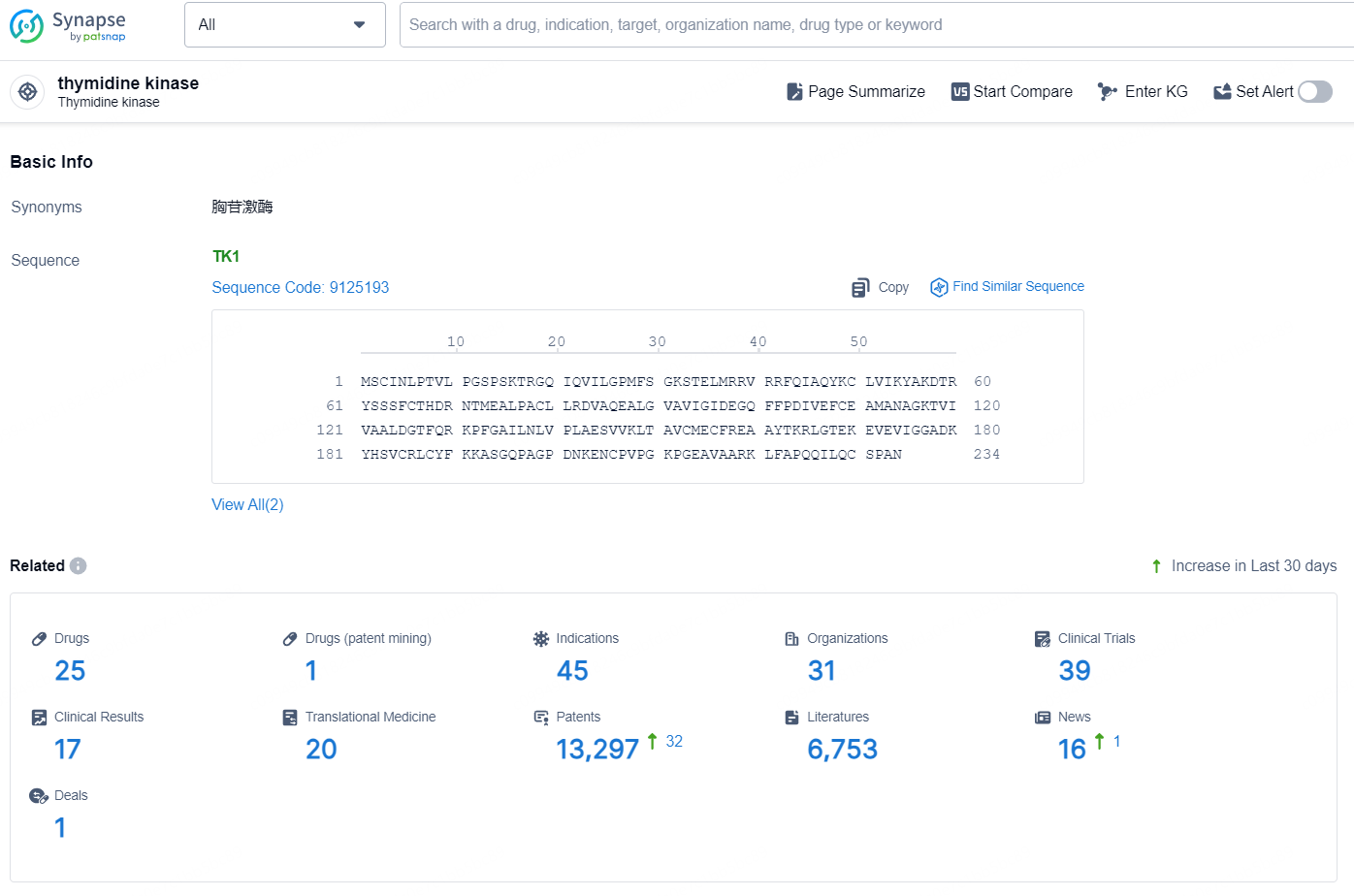
According to the data provided by the Synapse Database, As of December 18, 2024, there are 25 investigational drugs for the thymidine kinase target, including 45 indications, 31 R&D institutions involved, with related clinical trials reaching 39, and as many as 13297 patents.
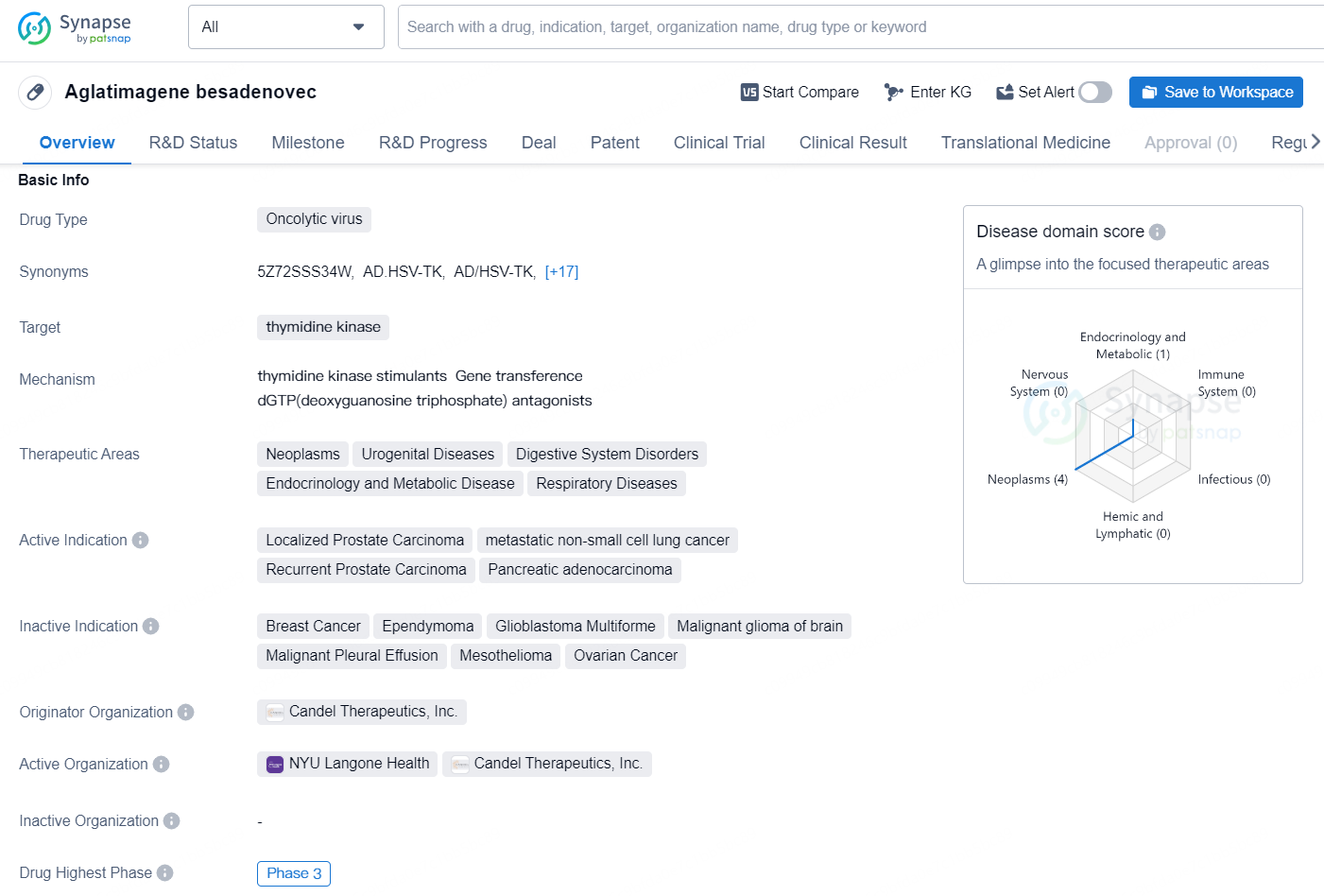
Aglatimagene besadenovec is an oncolytic virus drug that targets thymidine kinase and has therapeutic applications in diverse areas such as neoplasms, urogenital diseases, digestive system disorders, endocrinology and metabolic disease, and respiratory diseases. The active indications for this drug include localized prostate carcinoma, metastatic non-small cell lung cancer, recurrent prostate carcinoma, and pancreatic adenocarcinoma.