CASI Pharmaceuticals Proposes IND for CID-103 and Considers Sale of China Operations
CASI Pharmaceuticals, Inc., a biopharmaceutical firm focused on advancing and marketing novel therapeutic and pharmaceutical solutions, revealed today its intention to file an Investigational New Drug application with the U.S. Food and Drug Administration by the close of 2024. This application is for CID-103, aimed at treating antibody-mediated rejection in individuals who have undergone kidney transplants.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
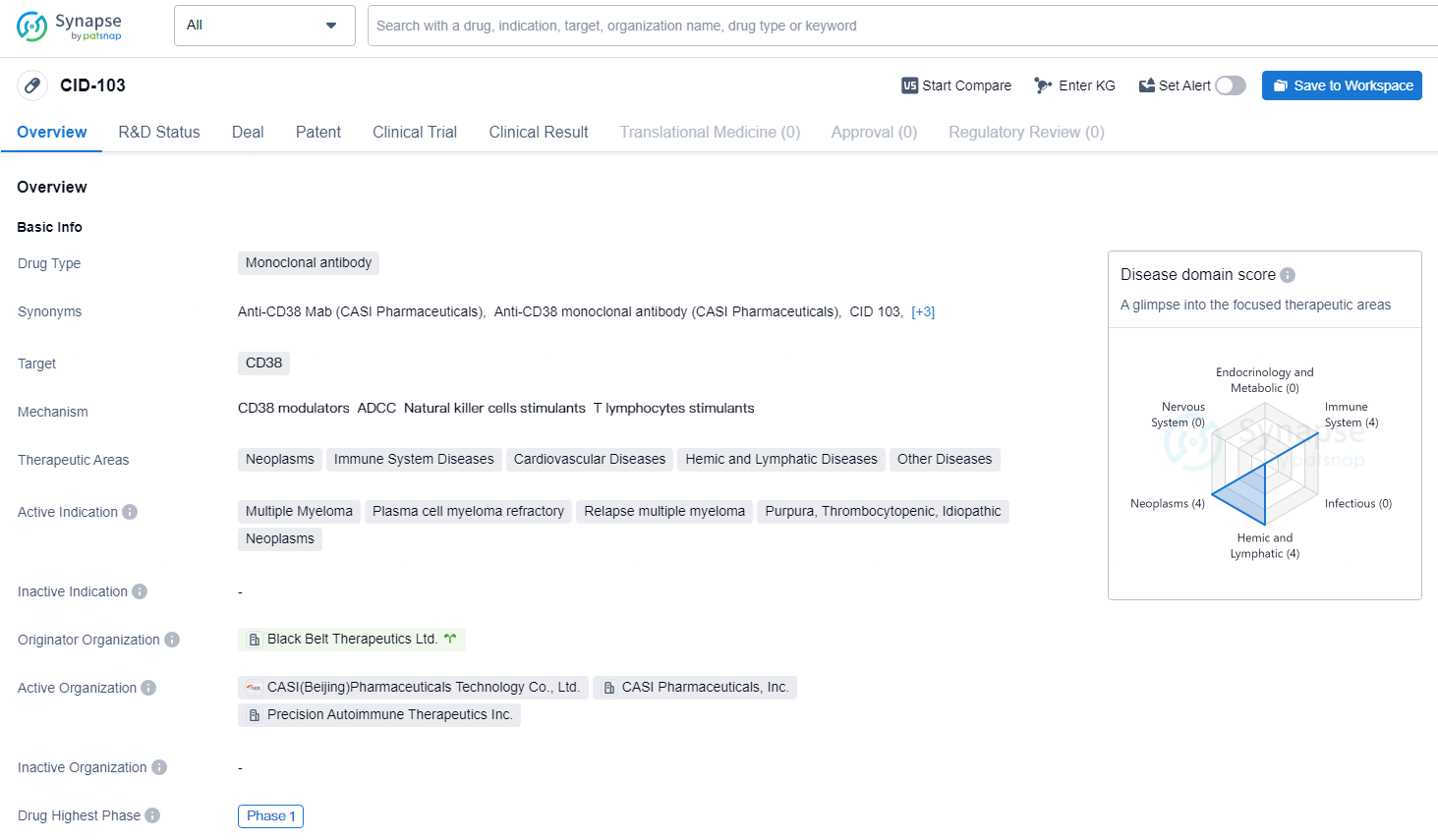
CID-103 is a fully human IgG1 anti-CD38 monoclonal antibody that uniquely targets a specific epitope. It has shown promising preclinical efficacy and safety when compared to other anti-CD38 monoclonal antibodies. The Company anticipates that the funds from the recently announced private placement financing, along with its current cash and cash equivalents, will be sufficient to complete the Phase II clinical trial for AMR.
Additionally, CASI disclosed that on June 21, 2024, its board of directors received a preliminary non-binding proposal from Dr. Wei-Wu He, Chairman of the Board and CEO, to purchase the Company's entire operations in China and all related licensing, distribution, and product rights in Asia. This includes but is not limited to EVOMELA, FOLOTYN, CNCT19, BI-1206, CB-5339, CID-103, and Thiotepa, for a total of $40.0 million, which would also cover the assumption of up to $20.0 million in Company debt.
On June 25, the Board established a special committee, consisting solely of current independent directors, to assess the transaction proposed in the letter from Dr. He and to explore other strategic and business alternatives for the Company's operations in China.
The Board and the Special Committee advise the Company's shareholders and potential investors that no decisions have been finalized regarding the Proposed Transaction or any other strategic options. There is no guarantee that a definitive offer will be made, that a final agreement will be signed in connection with the Proposed Transaction, or that any other transaction will be approved or completed. The Company does not commit to providing any updates on the transaction, except as required by relevant laws.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
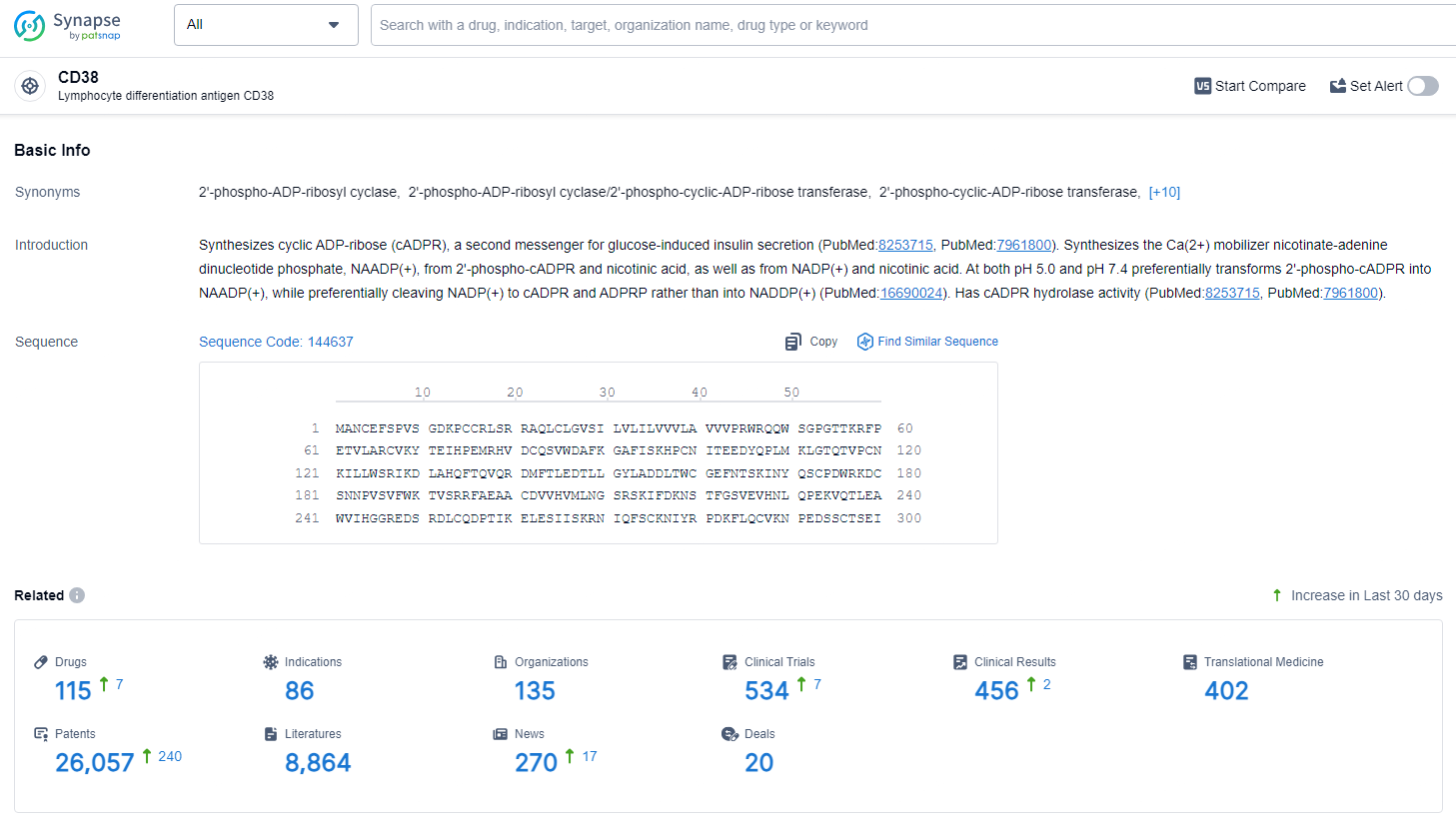
According to the data provided by the Synapse Database, As of July 2, 2024, there are 115 investigational drugs for the CD38 target, including 86 indications, 135 R&D institutions involved, with related clinical trials reaching 534, and as many as 26057 patents.
CID-103 is a monoclonal antibody drug that targets CD38. The drug is being investigated for its potential in treating a range of therapeutic areas, including neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, and other diseases. CID-103 has reached Phase 1 of clinical development globally. In China, the drug has received IND approval, indicating progress in the regulatory process for clinical trials in the country.