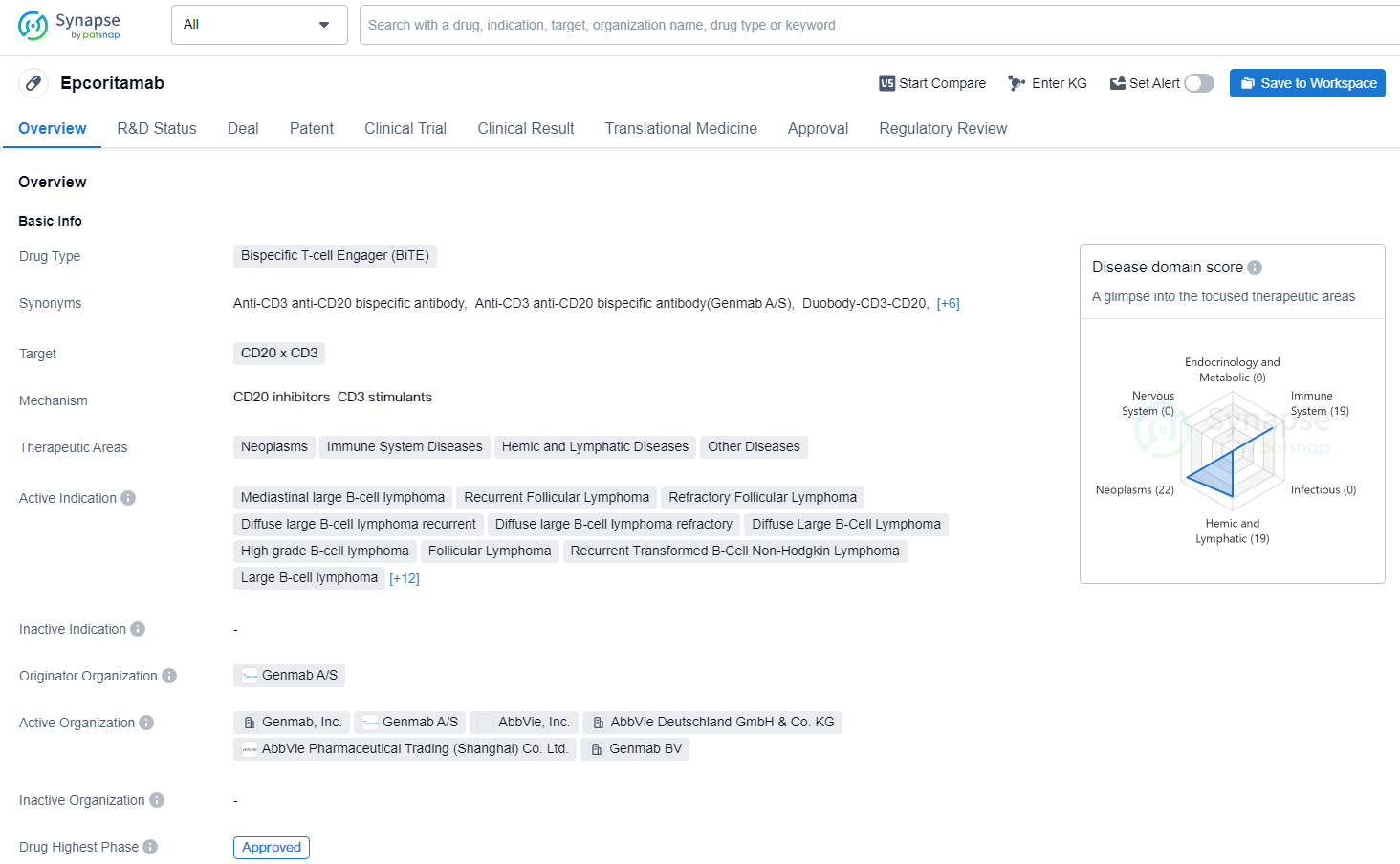
FDA Approves EPKINLY® for Relapsed/Refractory Follicular Lymphoma
Genmab A/S has disclosed that EPKINLY (epcoritamab-bysp) has received approval from the U.S. Food and Drug Administration for use in adult patients with relapsed or refractory follicular lymphoma following at least two prior systemic treatment regimens.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
With this approval, EPKINLY becomes the first and only T-cell engaging bispecific antibody administered subcutaneously approved in the United States for this patient group. This indication received accelerated approval based on response rate data. Ongoing approval may require confirmation and detailed demonstration of clinical benefits through further clinical trials.
Follicular Lymphoma (FL) is the second most prevalent type of non-Hodgkin's lymphoma, representing 20-30% of all NHL cases. Approximately 15,000 new cases of FL are diagnosed annually in the U.S. Current standard treatments are considered inadequate as FL is seen as incurable and often leads to relapse. With each subsequent therapy line, patients receiving current treatments may experience a decrease in response duration.
“Patients with relapsed or refractory follicular lymphoma face significant treatment hurdles, particularly in third-line settings where no definitive standard of care exists,” stated Jeff Sharman, MD, Disease Chair, Hematology Research, Sarah Cannon Research Institute at Willamette Valley Cancer Institute in Eugene, Oregon. “The approval and durable responses noted in the follicular lymphoma cohort during the EPCORE NHL-1 clinical trial, including real-world challenging cases, highlight EPKINLY's potential for patients with few remaining therapeutic options.”
“With this approval, patients with follicular lymphoma that has relapsed or is refractory to at least two prior systemic therapies now have the option to be treated with EPKINLY. This treatment has shown durable responses without the need for mandatory hospitalization, utilizing a three-step dosage regimen in clinical trials,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab.
“In a little over a year, EPKINLY has achieved a second indication in the U.S., making it the first and only bispecific antibody approved for patients with diffuse large B-cell lymphoma and follicular lymphoma after two or more prior systemic therapies. This, alongside the ongoing clinical development program, emphasizes epcoritamab's potential as a core treatment for B-cell malignancies,” Jan van de Winkel concluded.
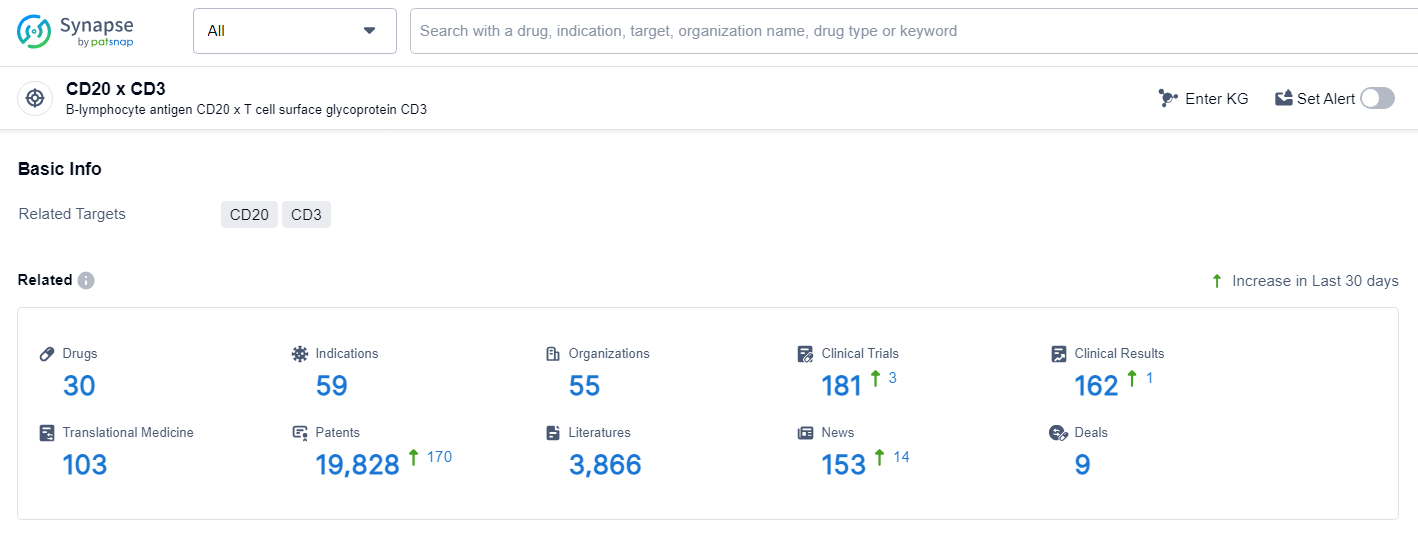
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 2, 2024, there are 30 investigational drugs for the CD20 and CD3 target, including 59 indications, 55 R&D institutions involved, with related clinical trials reaching 181, and as many as 19828 patents.
Epcoritamab's approval represents a significant advancement in the treatment of various types of lymphomas, providing new therapeutic options for patients with these conditions. The drug's mechanism of action as a bispecific T-cell engager targeting CD20 x CD3 demonstrates its potential to effectively engage the immune system in targeting cancer cells. The regulatory designations it has received further underscore the potential of Epcoritamab as a promising treatment option for patients with these challenging diseases.