CatalYm Secures $150M Funding for Extensive Phase 2b Trials of Visugromab
CatalYm has reported the successful closure of their Series D financing, raising $150 million. This round, which was oversubscribed, saw leadership from new investors such as Canaan Partners and Bioqube Ventures, along with participation from Forbion’s Growth Opportunities Fund, Omega Funds, and Gilde Healthcare. Returning investors including Jeito Capital, Brandon Capital Partners, Novartis Venture Fund, and Vesalius Biocapital III also contributed.
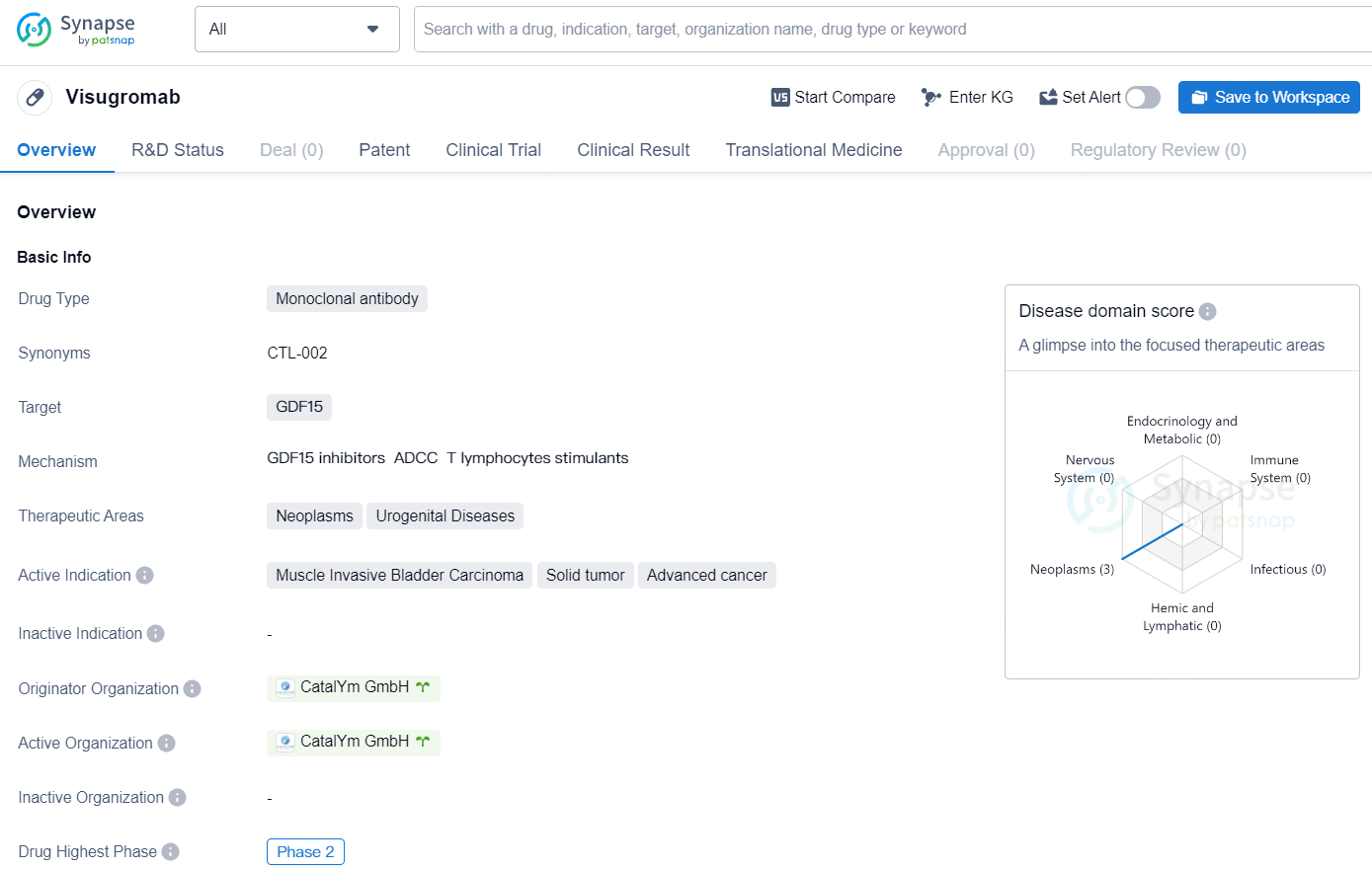
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The funds raised will support the company's broad expansion into Phase 2b development of visugromab, focusing on placebo-controlled Phase 2b trials in specific front-line and second-line treatment settings for checkpoint naïve patients. Visugromab has already shown excellent anti-tumor efficacy when combined with checkpoint inhibitor therapies.
As a humanized monoclonal antibody, visugromab targets and inhibits Growth Differentiation Factor-15 (GDF-15), a tumor-produced protein that plays a critical role in immune resistance against cancer treatments. CatalYm recently showcased compelling follow-up data from its ongoing "GDFATHER" Phase 1/2a study in an oral presentation at the 2024 Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago.
The results indicated that visugromab, when administered with the anti-PD-1 antibody nivolumab, achieved significant and lasting anti-tumoral effects, including several complete responses in patients with non-small cell lung cancer, urothelial cancer, or hepatocellular carcinoma who had relapsed or were refractory to anti-PD-1/PD-L1 treatment.
Following the conclusion of the financing round, Colleen Cuffaro from Canaan, Jon Edwards from Bioqube Ventures, and Otello Stampacchia from Omega Funds will join CatalYm's Board of Directors. Stefan Luzi from Gilde Healthcare will also join as a Board Observer.
Colleen Cuffaro of Canaan Partners stated: "The data from ASCO underscore visugromab's unique therapeutic potential and validate Phil and his team’s ability to swiftly advance the clinical plan. We look forward to providing strategic guidance as the company moves into Phase 2b, aiming to transform current treatment protocols for difficult-to-treat solid tumors."
Adding to this, Jon Edwards from Bioqube Ventures noted: "Initially, we were drawn to the intriguing biology of GDF-15 and found CatalYm to be at the leading edge of this area. We believe this method has the potential to markedly enhance the durability and depth of responses, unlocking the full potential of immuno-oncology therapies. We are enthusiastic about supporting this exceptional team and investor group in conducting rigorous clinical trials across a range of promising indications."
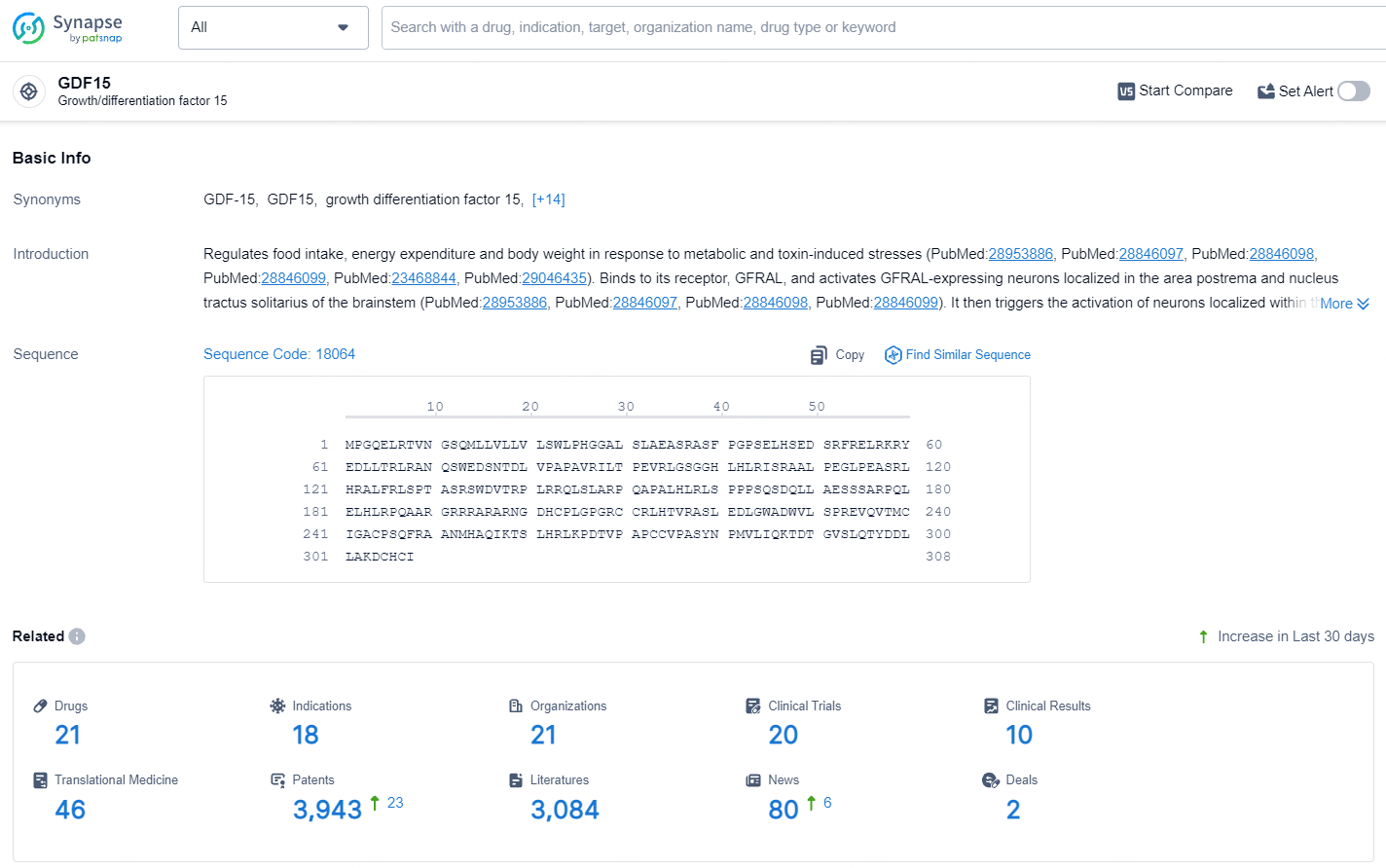
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 18, 2024, there are 21 investigational drugs for the GDF-15 targets, including 18 indications, 21 R&D institutions involved, with related clinical trials reaching 20, and as many as 3943 patents.
Visugromab is a promising monoclonal antibody drug designed to target GDF15 and treat various types of cancer, including muscle invasive bladder carcinoma, solid tumors, and advanced cancer. As it continues through the development process, Visugromab has the potential to offer new treatment options for patients in need of effective therapies for these challenging medical conditions.