Is Levoketoconazole approved by the FDA?
Yes, levoketoconazole (Recorlev) is FDA approved. The FDA approved Recorlev on December 30, 2021, for the treatment of endogenous hypercortisolemia in patients with Cushing's syndrome when surgery is not an option or has not been curative.
Uses
Levoketoconazole is specifically used for the following:
- Endogenous Hypercortisolemia in Cushing's Syndrome: It helps manage high cortisol levels in adults who either cannot undergo surgery or have not been cured by surgery.
Levoketoconazole is not intended for treating fungal infections.
Dosage and Administration
- Initial Dose: 150 mg orally twice a day.
- Maintenance Dose: The dosage can be increased by 150 mg daily, no more frequently than every 2 to 3 weeks, based on 24-hour urine free cortisol levels and patient tolerability until an adequate response is achieved.
- Maximum Dose: 1200 mg per day, administered as 600 mg twice a day.
Levoketoconazole can be taken with or without food. Regular blood tests are required to monitor liver function and blood electrolyte levels, and heart function is monitored using an electrocardiogram (ECG).
Side Effects
Common side effects of levoketoconazole include:
- Nausea, vomiting, stomach pain, upset stomach
- Low potassium levels
- Easy bleeding, easy bruising
- High blood pressure
- Headache
- Abnormal uterine bleeding
- Redness of the skin
- Tiredness
- Muscle and back pain
- Sleep problems
- Fluid retention
- Cold symptoms such as stuffy nose, sneezing, sore throat
Serious side effects that require immediate medical attention include:
- Fast or pounding heartbeats, shortness of breath, sudden dizziness
- Liver problems such as loss of appetite, stomach pain, tiredness, itching, dark urine, clay-colored stools, jaundice
- Decreased adrenal gland hormones, which may cause nausea, vomiting, stomach pain, loss of appetite, feeling tired or light-headed, muscle or joint pain, skin discoloration, craving salty foods
- Male-specific side effects: breast enlargement and erectile dysfunction
- Female-specific side effects: low desire for sex and mood changes
- Low potassium level symptoms: leg cramps, constipation, irregular heartbeats, increased thirst or urination, numbness or tingling, muscle weakness
- Severe lightheadedness or fainting
Warnings and Precautions
- Liver Problems: Levoketoconazole should not be used in individuals with liver problems.
- Heart Rhythm Disorders: It should be avoided in patients with a history of heart rhythm disorders or long QT syndrome.
- Drug Interactions: Levoketoconazole can interact with many other medications, including those that cause QT prolongation or affect liver function.
Pregnancy and Fertility
- Levoketoconazole may harm an unborn baby. Patients should inform their doctor if they are pregnant or plan to become pregnant.
- The medication may affect fertility in both men and women, making it harder to achieve pregnancy.
Conclusion
Levoketoconazole (Recorlev) is an FDA-approved medication for the treatment of high cortisol levels in adults with Cushing's syndrome when surgery is not feasible or has not been successful. It offers a crucial treatment option for managing endogenous hypercortisolemia in these patients. Regular monitoring and awareness of potential side effects and drug interactions are essential for the safe and effective use of levoketoconazole. Always consult a healthcare provider for personalized medical advice and treatment plans.
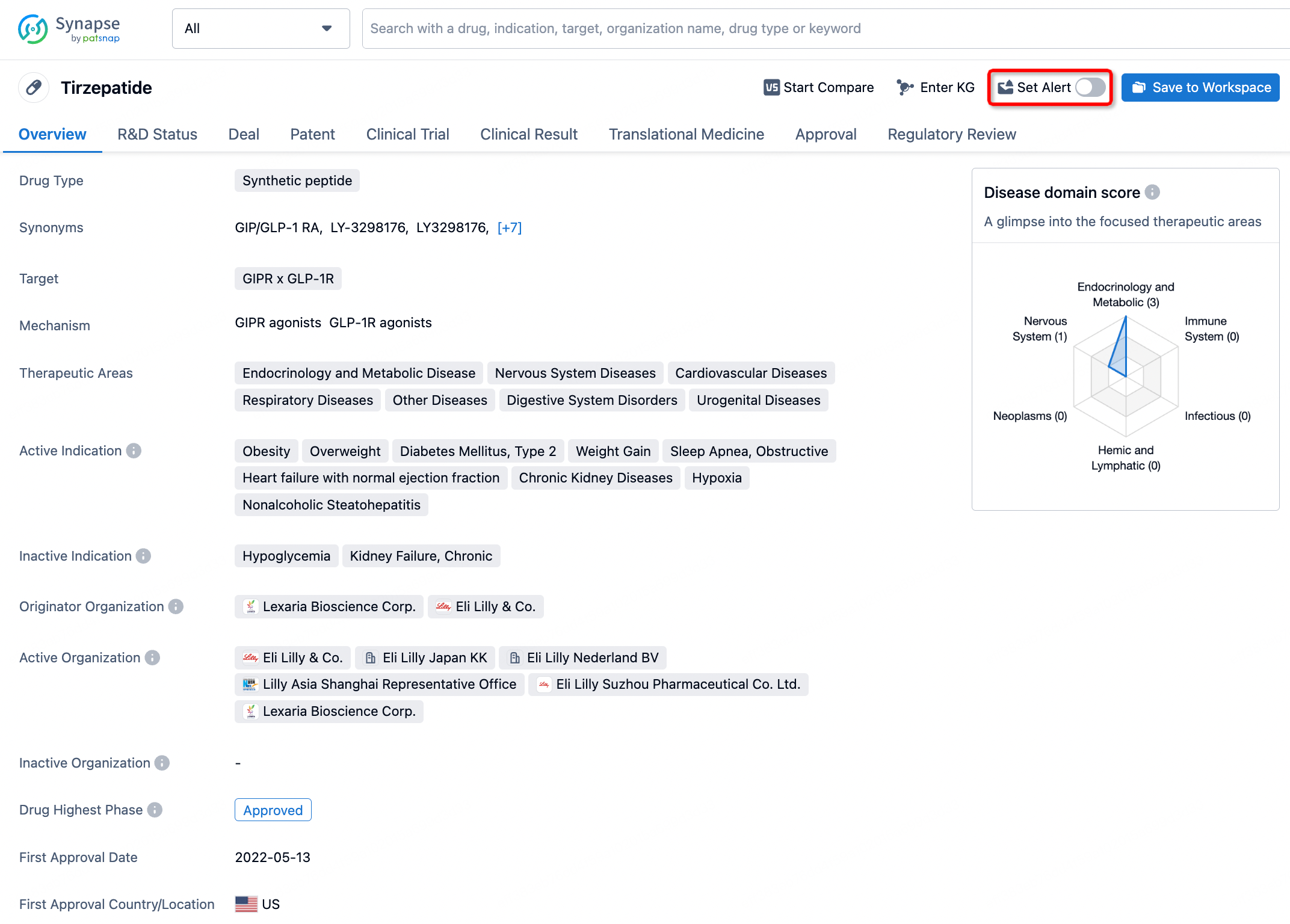
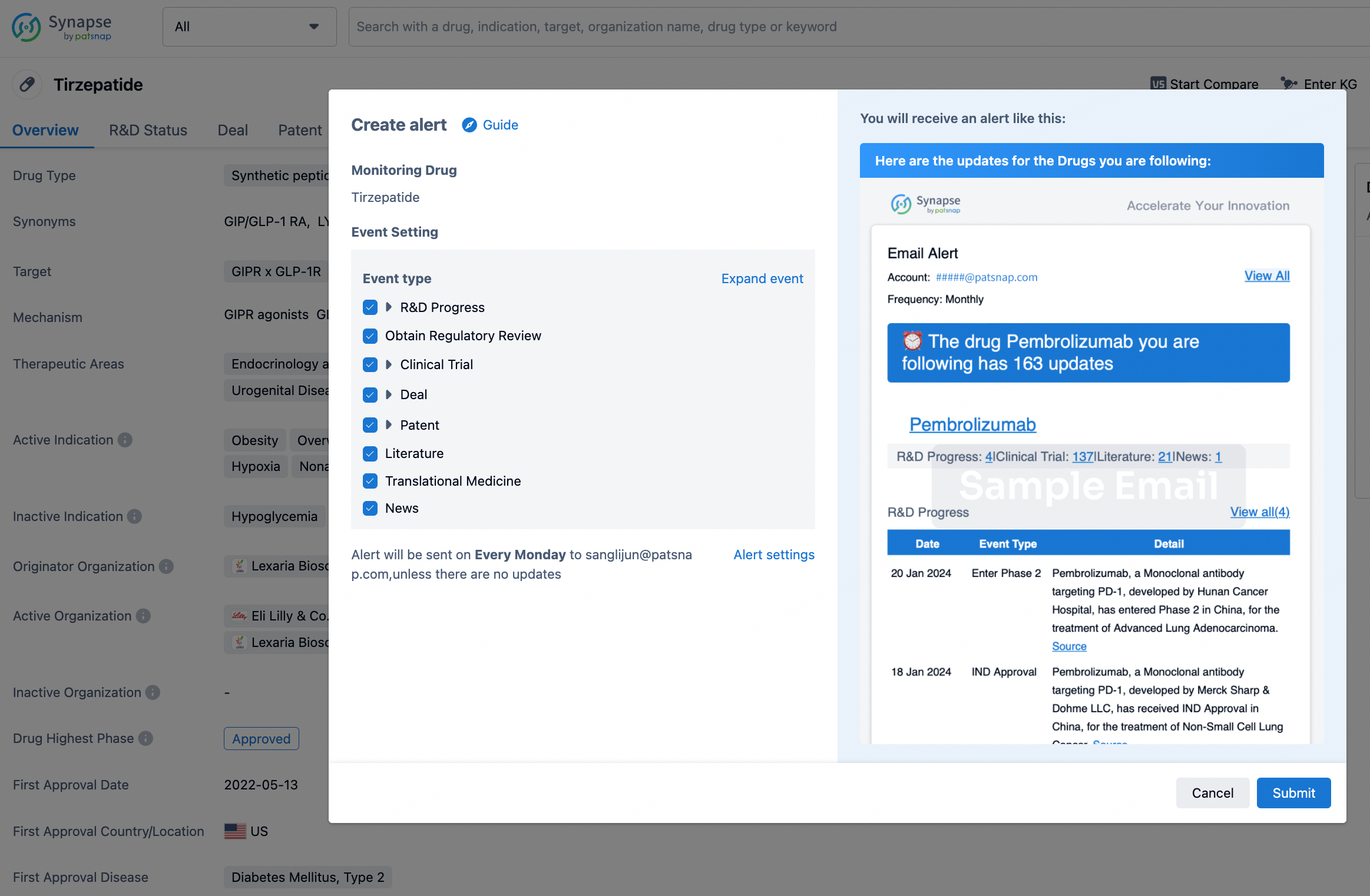
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!