The HERTHENA-Lung01 Phase 2 Trial demonstrated significant, lasting effects of Patritumab Deruxtecan in non-small cell lung cancer patients
Findings from the HERTHENA-Lung01 phase 2 investigation reveal that, following their disease advancement with an EGFR TKI and platinum-infused chemotherapy, patritumab deruxtecan (HER3-DXd) offered notable and long-lasting effects on patients with EGFR-mutated locally advanced or metastatic non-small cell lung cancer. The research was delivered as an oral presentation at the 2023 Global Lung Cancer Conference.
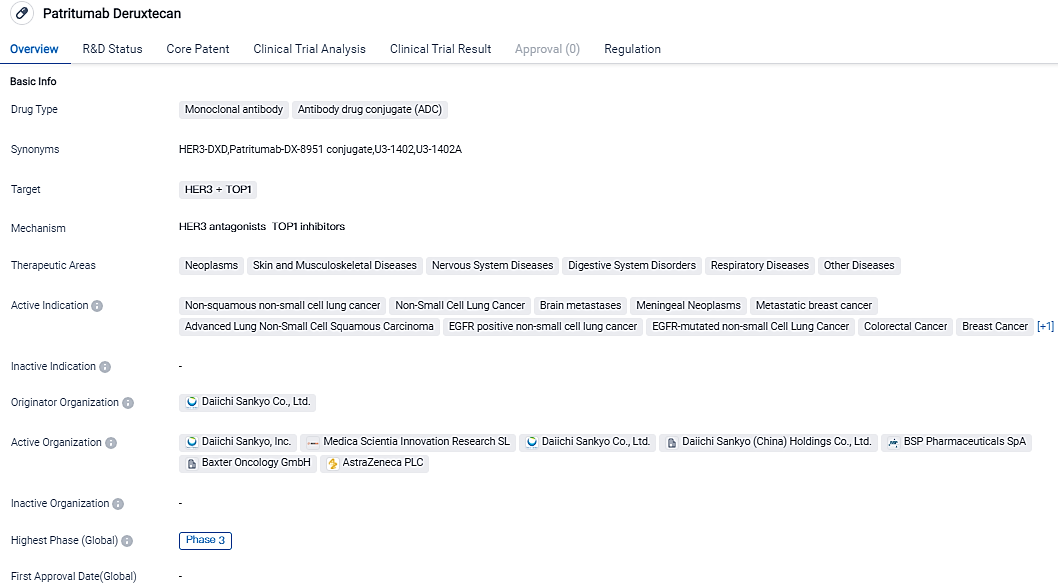
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Patritumab deruxtecan represents a meticulously formulated prospective first-in-class in the HER3 directed antibody drug conjugate group, developed utilizing Daiichi Sankyo’s unique DXd ADC technology.
Non-small cell lung cancer represents nearly 85% of all lung cancer incidences worldwide. At the time of diagnosis, 55% of these cases have already metastasized distantly. Among all cases, EGFR-activating mutations are identified in 14% to 38% of all NSCLC tumors. After the disease evolves post-treatment with an EGFR TKI and platinum-based chemotherapy, the available treatment options offer minimal efficacy, underlining the necessity to explore novel methodologies for better patient outcomes.
The consistent efficacy outcomes, including a sub-group of 209 patients who had previously gone under third-generation EGFR TKI and platinum-based chemotherapy treatments were observed. Anti-tumor activities with patritumab deruxtecan were also noted across varied mechanisms of EGFR TKI resistance and throughout a spectrum of pretreatment tumor HER3 membrane expression levels.
"The results obtained from HERTHENA-Lung01 furnished considerable support to the effectiveness of patritumab deruxtecan in patients with advanced EGFR-mutated non-small cell lung cancer," stated Helena Yu, MD, an Associate Attending Physician at Memorial Sloan Kettering Cancer Center.
"Disease progress is a certainty in patients who have been previously treated and recurred metastatic EGFR-mutated non-small cell lung cancer, thereby strengthening the demands for novel and innovative treatment methodologies encompassing diverse mechanisms of resistance," remarked Ken Takeshita, MD, Global Head, R&D, Daiichi Sankyo. "These results will serve as a pillar to our ongoing discussions with health authority bodies including our future submission plans in the U.S."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
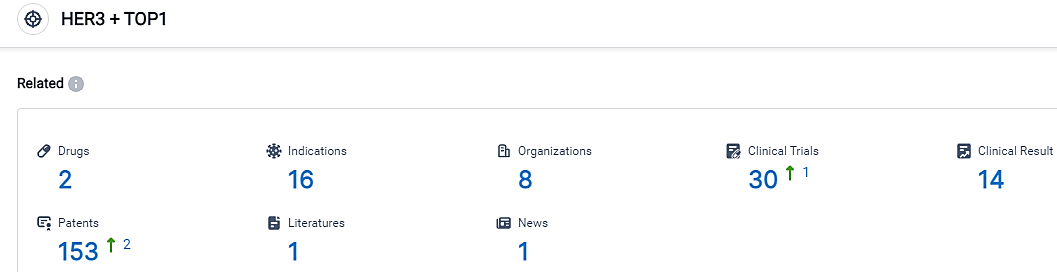
According to the data provided by the Synapse Database, As of September 14, 2023, there are 2 investigational drugs for the HER3 and TOP1 target, including 16 applicable indications,8 R&D institutions involved, with related clinical trials reaching 30,and as many as 153 patents.
Patritumab deruxtecan is currently being evaluated as both a monotherapy and in combination with other therapies in a global development program. Patritumab deruxtecan was granted Breakthrough Therapy Designationby the U.S. FDA in December 2021 for the treatment of patients with EGFR-mutated locally advanced or metastatic NSCLC with disease progression on or after treatment with a third-generation TKI and platinum-based therapies.