Celltrion USA Finalizes Application for Biosimilar Approval, Targets CT-P47 (ACTEMRA® Counterpart)
Celltrion USA has declared that they have officially filed a Biologics License Application with the U.S. Food and Drug Administration concerning CT-P47. This product is a biosimilar in development, formulated to mimic the therapeutic effects of the original drug ACTEMRA® (tocilizumab).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The application for regulatory consideration concerning CT-P47 was constructed on the foundation of insights gathered from a sizable international Phase III study. The investigation's purpose was to ascertain the treatment's effectiveness, pharmacokinetic attributes, safety profile, and potential for generating an immune response when compared against the benchmark medication ACTEMRA®. The focus was on individuals experiencing a moderate to severe degree of active rheumatoid arthritis, particularly those who had not adequately responded to methotrexate treatment, with data collected up to the 52-week mark.
Commenting on this development, Celltrion USA's Chief Commercial Officer, Thomas Nusbickel remarked, "Introducing CT-P47 into the regulatory review stage marks a pivotal step in providing those affected by rheumatoid arthritis a more attainable option for managing a condition that exerts a considerable health impact. Our ambition is to navigate the U.S. market for autoimmune disease therapies by forging a broad-ranging portfolio. Our ongoing efforts include working collaboratively with the FDA to expedite the availability of CT-P47 to individuals affected by rheumatoid arthritis."
Thomas Nusbickel further elaborated on the strategy, "By pioneering in the autoimmune sector with a varied selection of products, our aim is to serve those affected by rheumatoid arthritis swiftly and effectively."
ACTEMRA® is currently approved to treat several conditions, including moderately to severely active rheumatoid arthritis among adults and pediatric conditions such as juvenile idiopathic polyarthritis and systemic juvenile idiopathic arthritis.
As for the therapeutic candidate CT-P47, this pharmaceutical agent, infused with tocilizumab, a key ingredient, is a humanized monoclonal antibody engineered to target and neutralize the activity of the interleukin 6 (IL-6) receptor. In pursuit of authorization, Celltrion is promoting the availability of CT-P47 for administration through both intravenous and subcutaneous routes.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
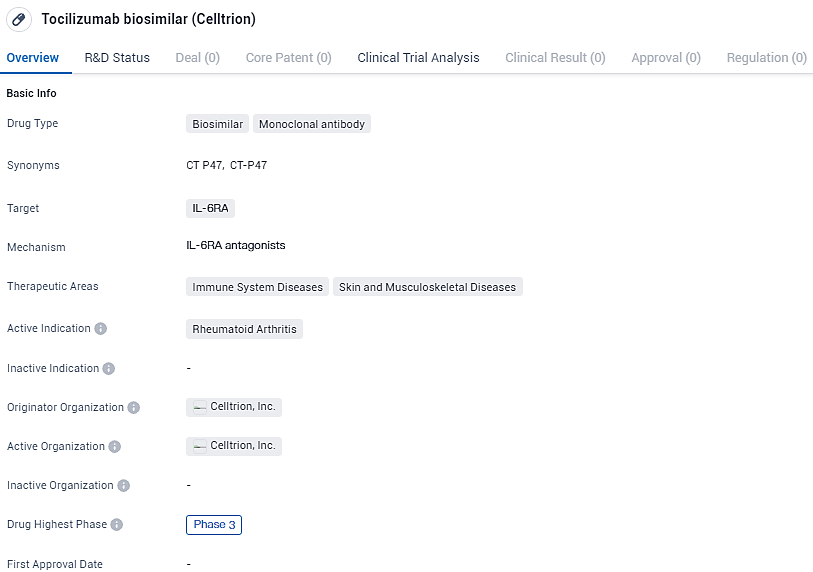
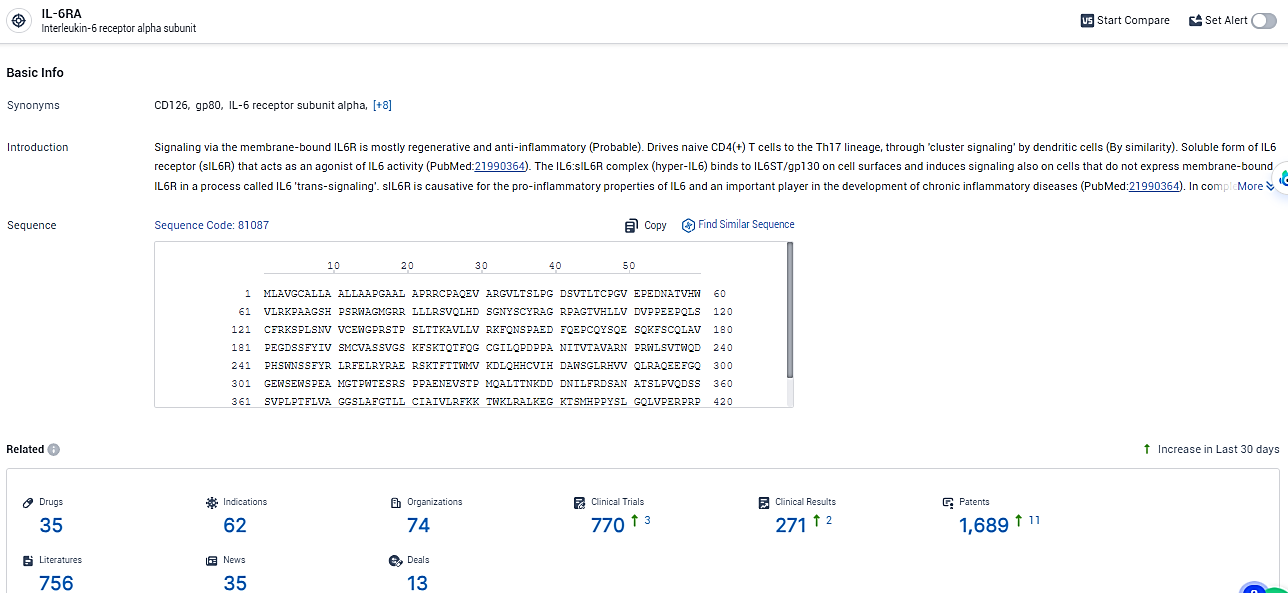
According to the data provided by the Synapse Database, As of February 2, 2024, there are 35 investigational drugs for the IL-6RA target, including 62 indications,74 R&D institutions involved, with related clinical trials reaching 770, and as many as 1689 patents.
CT-P47 is a monoclonal antibody drug that targets IL-6RA. It is primarily indicated for the treatment of Rheumatoid Arthritis and has shown potential in immune system diseases and skin and musculoskeletal diseases. With its highest phase being Phase 3, the drug has undergone extensive clinical testing and evaluation. The development of Tocilizumab biosimilar represents a significant advancement in the pharmaceutical industry, offering potential benefits in terms of improved patient access and cost savings.