Celltrion USA Submits Biosimilar Licensing Application for CT-P39, an Alternative to XOLAIR
Celltrion USA publicized its formal request for authorization of CT-P39, a proposed equivalent biosimilar for XOLAIR® (omalizumab), by filing a Biologics License Application with the U.S. Food and Drug Administration.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We're gratified by the swift advancement of CT-P39 and we're enthusiastic about broadening our range of products into new areas beyond our current focus on immunology and oncology," remarked Thomas Nusbickel, Chief Commercial Officer of Celltrion USA. "Our commitment to advancing a legacy of excellence in the production and development of therapeutic biosimilars remains unwavering, as this marks a significant stride in bettering patient care."
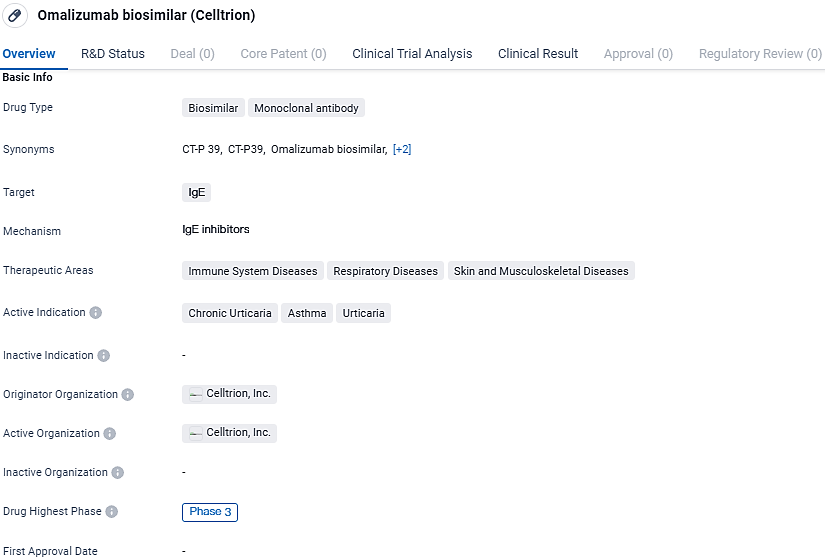
The submission for the Biologics License Application (BLA) encompasses data from a comprehensive international Phase III study aimed at assessing CT-P39's effectiveness, tolerability, and pharmacokinetic profile relative to the original therapy, XOLAIR®, in individuals dealing with chronic spontaneous urticaria for a duration of up to 40 weeks. Celltrion unveiled the initial findings from their 12-week study phase during the November 2023 conference of the American College of Allergy, Asthma and Immunology held in Anaheim, California.
Celltrion USA's application for CT-P39 seeks approval for all the therapeutic uses that XOLAIR®, a biologic medication administered through injection, currently holds. These indications include treatment for asthma, chronic rhinosinusitis with nasal polyps, IgE-mediated food allergies, and chronic spontaneous urticaria (CSU).
Market analysis by IQVIA, a pharmaceutical market intelligence firm, reveals that XOLAIR® reached a staggering $3.89 billion in worldwide sales during 2022. The protection through its compound patent has already come to an end, with the formulation patent on course to expire in November 2025 in the United States.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
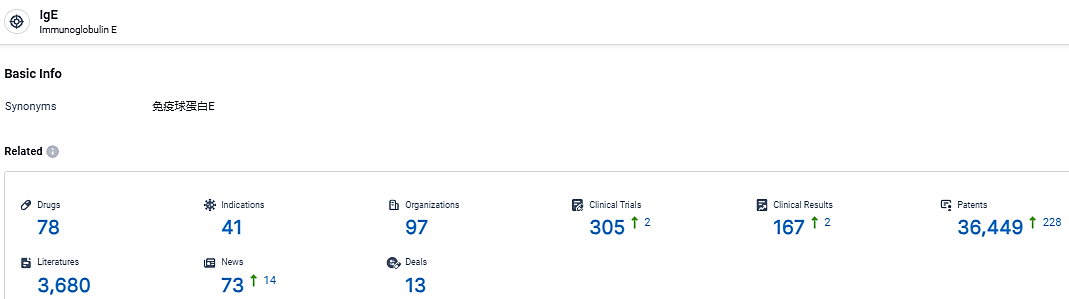
According to the data provided by the Synapse Database, As of March 11, 2024, there are 78 investigational drugs for the IgE target, including 41 indications, 97 R&D institutions involved, with related clinical trials reaching 305, and as many as 36449 patents.
CT-P39 is a monoclonal antibody drug that targets IgE and is used in the treatment of immune system diseases, respiratory diseases, and skin and musculoskeletal diseases. Its active indications include chronic urticaria, asthma, and urticaria. Celltrion, Inc. is the originator organization behind the drug, and it has reached Phase 3 of clinical development.