Wegovy® Approved in the US for Heart Health in Obesity
Novo Nordisk has disclosed the authorization of an expanded use label for Wegovy® by the US Food and Drug Administration. This approval is in response to a submitted supplemental New Drug Application. It highlights the drug's efficacy in lowering the incidence of significant cardiovascular complications. These complications comprise fatal and non-fatal cardiac incidents, as well as non-fatal cerebrovascular incidents, in individuals presenting with excessive weight or obesity concurrent with a history of cardiovascular disorders.
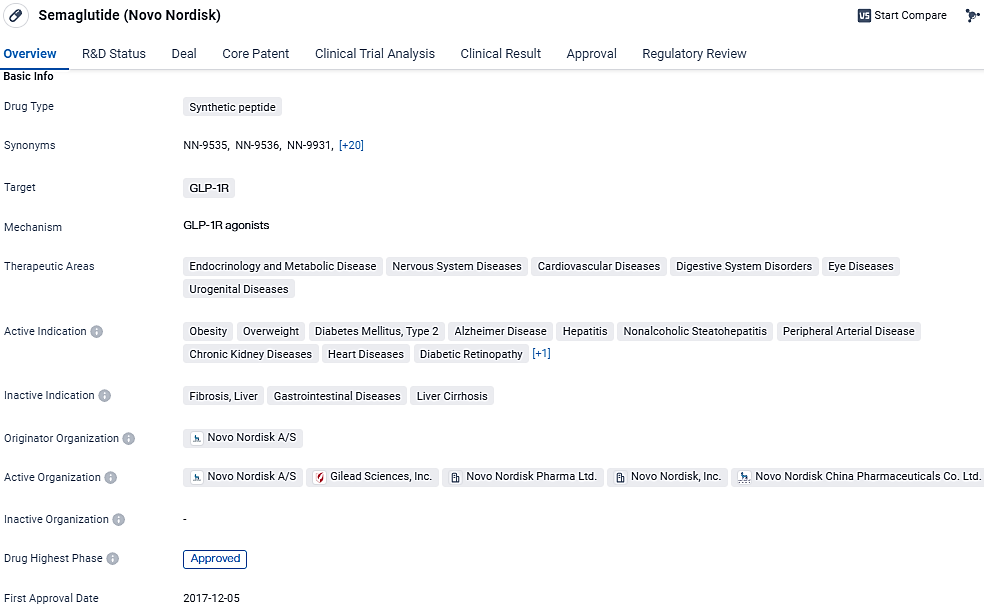
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The sanction of the medication comes following findings from the SELECT cardio-specific outcomes study. This research indicated that Wegovy® notably diminished the hazard of major adverse cardiovascular events (MACE) by a fifth relative to a non-active treatment when combined with standard care practices. The precise methodology behind the lessening of cardiovascular peril remains to be clarified.
Data derived from the SELECT investigation reveal that, throughout a timespan of up to half a decade, mitigation in MACE occurrence persisted irrespective of initial factors such as age, gender, racial or ethnic origins, body mass index, or degree of kidney function compromise. Furthermore, updated information included in the medication's indications showcase that SELECT identified a cardiovascular mortality hazard diminution of 15%, and an all-cause mortality hazard lowering of 19%, both in comparison to the non-active treatment. The medication's documentation now also encompasses auxiliary details from the SELECT study.
“We're immensely satisfied that Wegovy® has gained clearance in the United States as the pioneering treatment for individuals aiming to govern their weight and diminish cardiovascular threats,” expressed Martin Holst Lange, the chief officer of Development at Novo Nordisk.
Holst Lange added, “This clearance represents a critical achievement for individuals grappling with obesity and heart-related conditions, given that SELECT outcomes suggest that Wegovy® could extend lifespans through mitigating the prevalence of events leading to death that could be prevented by curbing cardiovascular event risks.”
Novo Nordisk has progressed with requests to broaden the medication's labeling within the European Union, with anticipations of a conclusion in 2024.
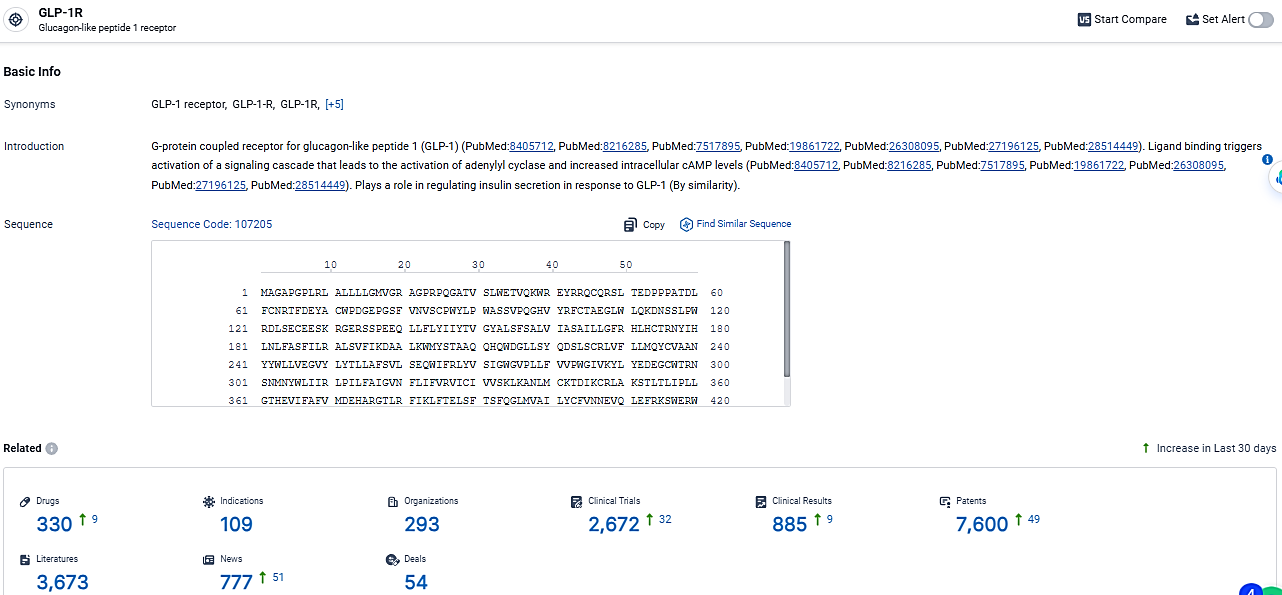
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 11, 2024, there are 330 investigational drugs for the GLP-1R target, including 109 indications, 293 R&D institutions involved, with related clinical trials reaching 2672, and as many as 7600 patents.
semaglutide targets the GLP-1R receptor and has been approved for various therapeutic areas, including endocrinology and metabolic disease, nervous system diseases, cardiovascular diseases, digestive system disorders, eye diseases, and urogenital diseases. Semaglutide received its first approval in the United States in December 2017 and has also been approved in China. Its breakthrough therapy designation highlights its potential to address unmet medical needs.