Celltrion's Infliximab (subcutaneous formulation) receives FDA approval for the treatment of inflammatory bowel disease
On October 25, 2023, Celltrion announced that the U.S. FDA has approved Zymfentra (infliximab) for maintenance treatment of adult patients with moderate to severe active ulcerative colitis (UC) and Crohn's disease (CD) after intravenous infliximab treatment. According to the press release, Zymfentra is the first infliximab subcutaneous formulation approved by the FDA for the treatment of inflammatory bowel disease (IBD).
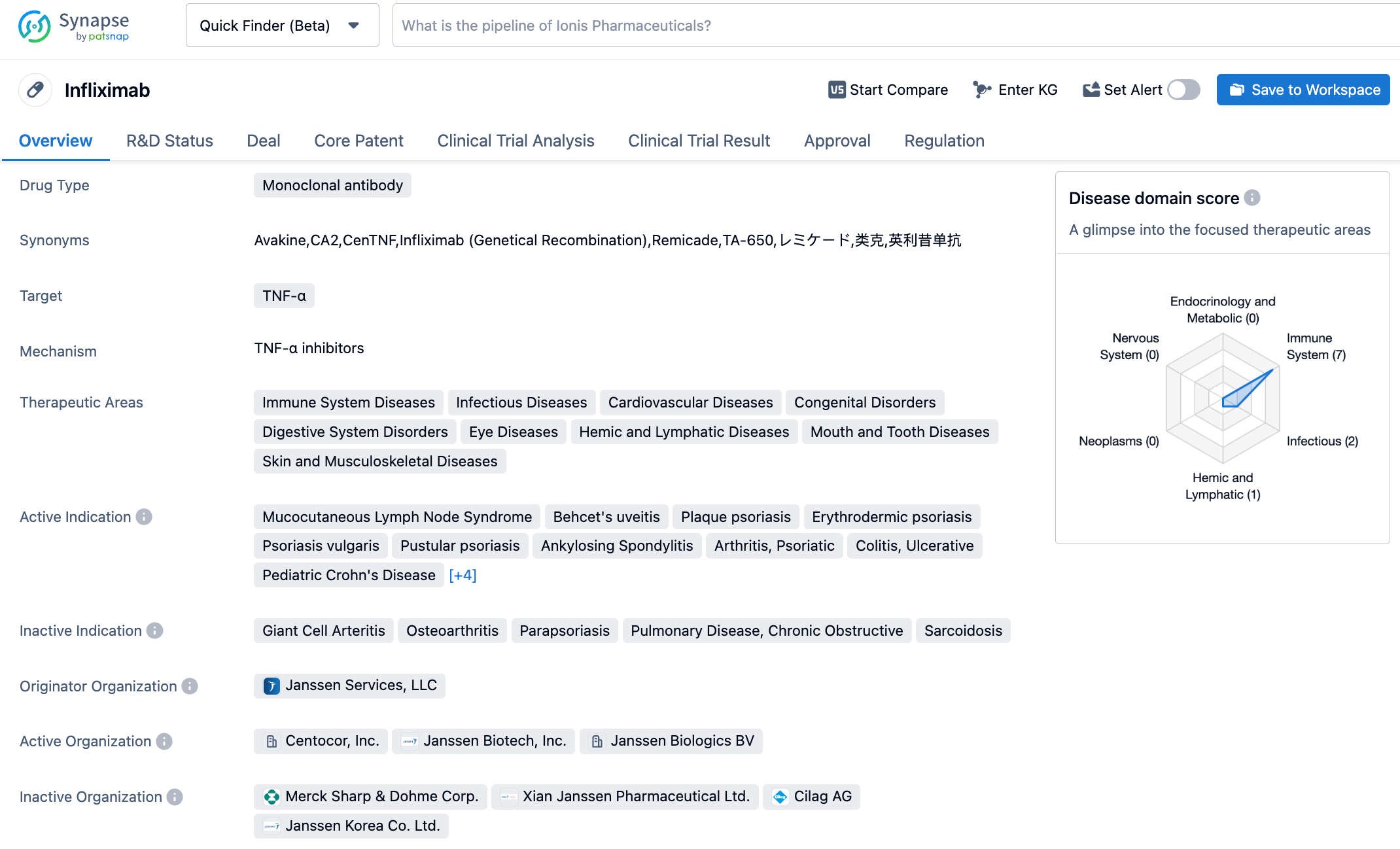
Infliximab (originally known as Remsima) was developed by Johnson & Johnson and is a monoclonal antibody against tumor necrosis factor (TNF-α), used to treat patients with CD, UC, rheumatoid arthritis and ankylosing spondylitis. Zymfentra is the first biosimilar developed by Celltrion, currently approved for moderate to severe IBD that is ineffective with routine treatment, and is one of the four main biological drugs for treating IBD.
The subcutaneous formulation of Zymfentra can provide stable elevated levels of infliximab in the serum. The FDA approval of Zymfentra was primarily based on data from Phase III trials evaluating the efficacy and safety of Zymfentra as maintenance treatment for patients with moderate to severe UC (LIBERTY-UC) and CD (LIBERTY-CD). Results from LIBERTY UC and LIBERTY CD studies showed that after 54 weeks of research, compared to placebo, Zymfentra demonstrated superior efficacy in achieving the primary endpoint of clinical remission (for both UC and CD) and endoscopic remission (for CD). In both studies, the overall safety profile of Zymfentra during the maintenance period was similar to placebo and no new safety signals were observed.
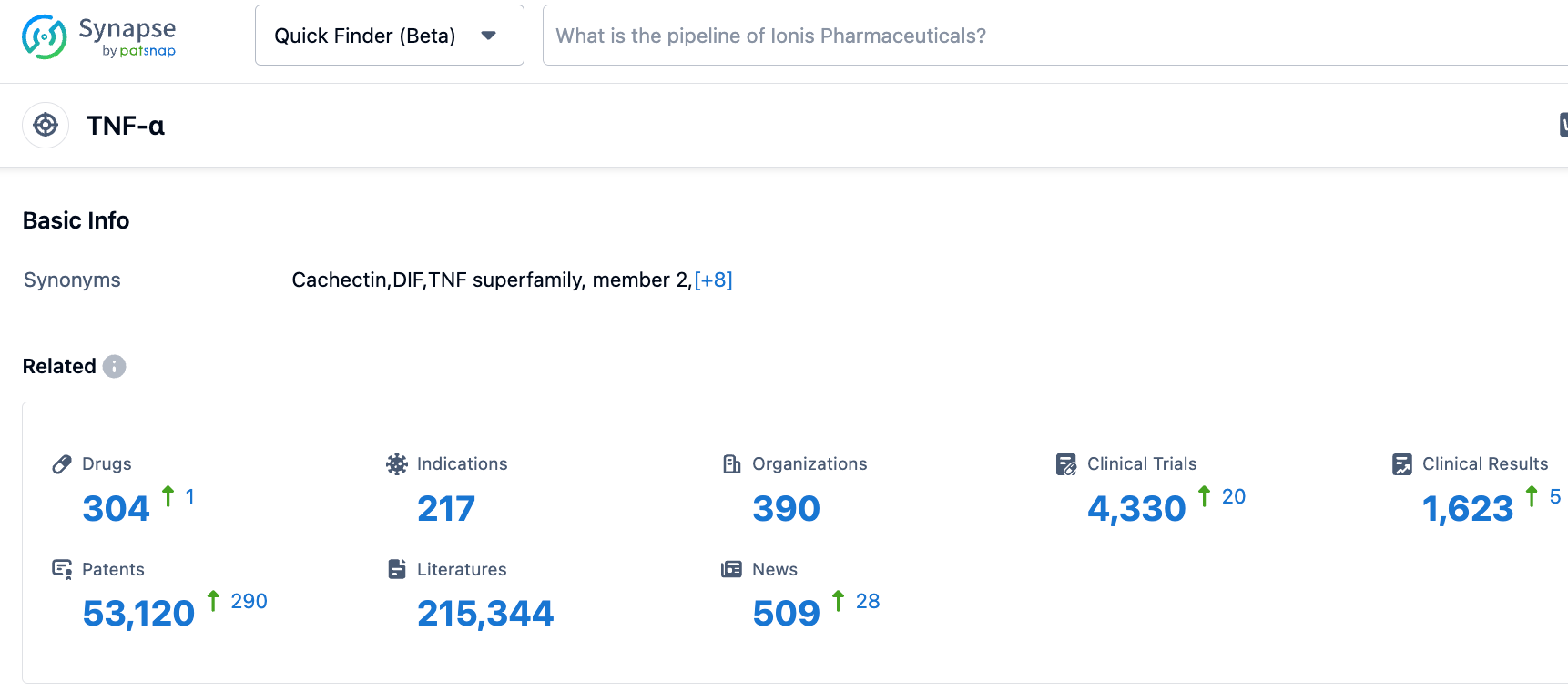
According to information disclosed by the Synapse Database, as of October 26, 2023, there were 304 investigative drugs targeting TNF-α, covering 217 indications, under investigation by 390 institutions, involving 4329 clinical trials, and 53112 patents in total. The subcutaneous formulation of Zymfentra improves the shortcomings of the original intravenous administration, enabling patients to self-inject, reducing the number of hospital visits, and improving the convenience of medication. Meanwhile, the new formulation offers physicians an alternative choice of administration, overcoming the limitations of TNF-α inhibitors and bringing benefits to more patients.