Merck reports the use of LRRK2 inhibitor for Parkinson's treatment
In 1668, Friedrich Jacob Merck (1621-1678) acquired the Angel's Apothecary in Darmstadt, launching the development route of the enterprise. The Angel's Apothecary later became the origin of the Merck Company. Over five centuries, Merck eventually evolved into an MNC pharmaceutical giant. In 2022, Merck reported a total revenue of 58.47 billion U.S. dollars.
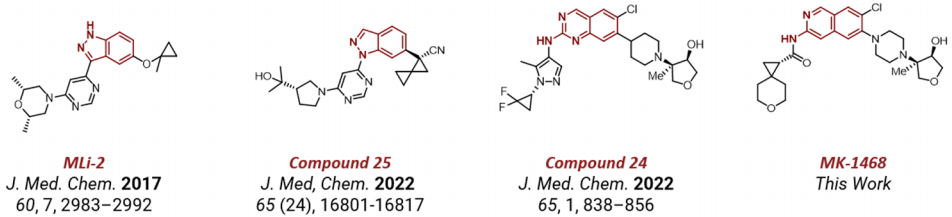
Merck Company published an article in the Journal of Medicinal Chemistry (JMC) on the 20th of this month, reporting on the discovery process of LRRK2 inhibitor MK1468. As early as 2017, Merck had reported on this type of imidazole series of inhibitors. After more than six years, Merck's R&D personnel continuously optimized and improved it, sequentially discovering compounds 25 and 24 and recently reporting on the clinical candidate molecule MK-1468. These kinds of inhibitors are mainly used for the treatment of Parkinson's disease.
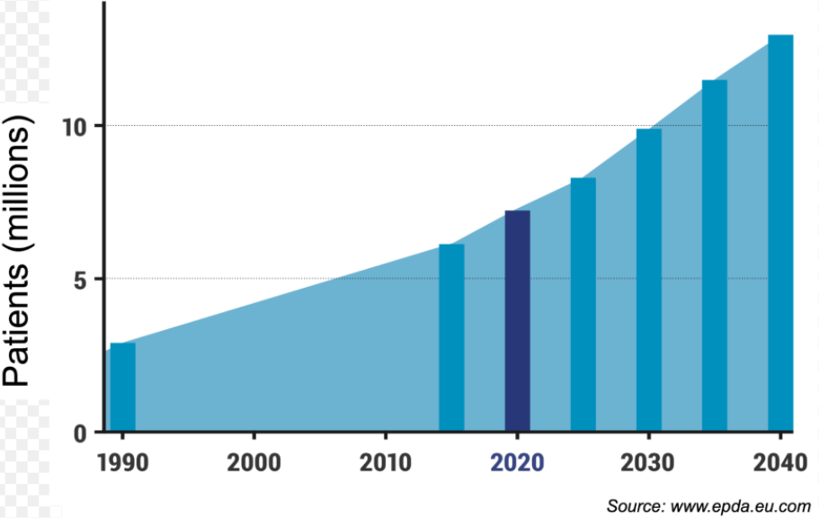
Parkinson's Disease (PD) is a chronic progressive neurodegenerative disorder, affecting approximately 1 million people in the United States and over 6 million globally, projected to surpass 10 million by 2040. The hallmark features of PD are the gradual loss of dopamine neurons in the substantia nigra and accumulation of α-synuclein-rich protein deposits, known as Lewy bodies. Common symptoms include resting tremors, slow movement, muscle stiffness, and postural instability. Current approved PD therapies include Levodopa, Dopamine and Monoamine Oxidase inhibitors.
These treatment options often come with adverse events (AEs) such as nausea, impulsivity/compulsive behaviors, and hallucinations. Moreover, these treatments do not affect disease progression. There is an unmet need for novel drugs with clinical significance.
The Discovery of MK1468
Missense mutations in Leucine-rich repeat kinase 2 (LRRK2) are the most common cause of autosomal dominant PD, accounting for about 1-3% of sporadic PD cases globally and 5-6% of familial PD cases. Increased kinase activity of nigrostriatal dopamine neurons was observed in the postmortem brain tissue of sporadic PD patients. These data also support the hypothesis that inhibiting LRRK2 kinase activity could benefit sporadic and/or LRRK2 PD patients.
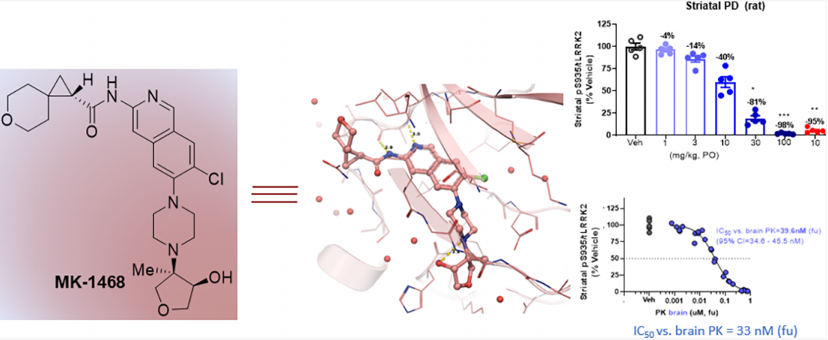
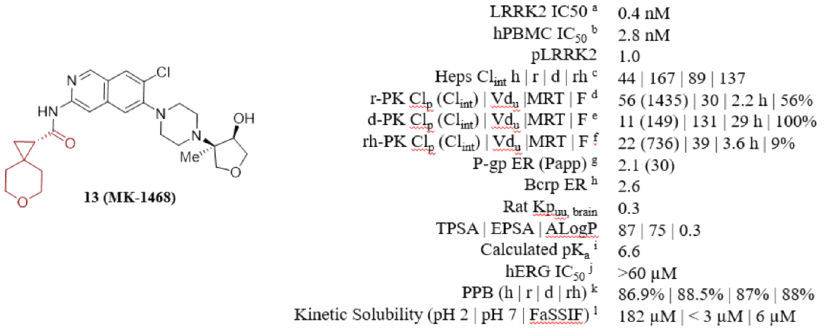
MK1468 is a leucine-rich repeat kinase 2 (LRRK2) inhibitor derived from amino isoquinoline (IQ), developed based on Merck's previous LRRK2 inhibitors. It reduces lipophilicity and basicity, thereby weakening the inhibition of hERG ion channels, while maintaining favorable extracranial neurotransmitter transporter characteristics. Further structure and property-based optimization led to the discovery of MK1468. The anticipated human dose is 48mg BID, and its preclinical safety has been supported by GLP toxicology studies.
Compared to compound 24, MK-1468 incorporates piperazine nitrogen. The observed pKa reduction through the addition of piperazine nitrogen contributes to slight improvements in P-gp efflux and BCRP activity maintenance. The rat and dog PK data for MK1468 was good, exhibiting bioavailabilities of 56% and 100%, respectively. These data highlight encouraging EDE doses (BID 15-20mg, allometric scaling – IVIVE), prompting further research.
Compound MK-1468 was subjected to kinase inhibitory testing against 267 protein kinases, including LRRK2 (G2019S) and wild-type LRRK2, exhibiting nearly equivalent potency among these protein kinases. MK1468 shows 100-fold selectivity for 265 off-target kinases. Additionally, it exhibits 1000-fold selectivity for 117 screened functional assays, enzymatic and radioligand binding tests (Cerep, Panlabs). These data suggest MK-1468 is one of the most selective type I (ATP-competitive) LRRK2 inhibitors studied by Merck.
Merck is also advancing preclinical candidate studies of MK-1468, including determined PK, PK/PD, and in vivo safety studies. Although the bioavailability in rats and dogs was high, relatively shorter MRT (3.6 h) and lower F% (9%) were observed in cynomolgus monkeys. Merck attributed the low bioavailability of the compound in cynomolgus monkeys to relatively lower thermodynamic solubility and higher first-pass extraction rates. Using a method combining IVIVE and three allometric scaling measurements, the expected human dose is 18 mg BID with an effective half-life of 9.3 h in humans.
Then, in vivo PK / PD experiments were carried out on rats to assess the ability of this compound to inhibit the phosphorylation of LRRK2 serine 935 (pSer935) in rat brain. MK-1468 was administered to male rats aged 8-10 weeks at doses of 1, 3, 10, 30, and 100 mg / kg (PO) respectively, and tissue was collected for pharmacokinetic (plasma, cerebrospinal fluid, and brain) and pharmacodynamics (striatum) analysis 2 hours later. MK-1468 showed a dose-dependent reduction in pSer935 in the rat striatum (in vivo brain unbound IC50 = 33 nM), consistent with the unbound potency in rat ex vivo PBMCs (IC50 = 25 nM), Kpu,u = 0.3. Recognizing the potential biological distribution gradient in the clinic, Merck increased the dose by three times. This resulted in a revised dose estimate of 48mg BID (assuming Kpu,u = 0.3), with exposure targets as follows: AUCtau = 3.0 μM•h (AUC0−24h = 6.0 μM•h), Cmax = 0.75 μM, Cmin = 0.13 μM. These estimates will be further refined after additional Kpu,u data is generated in preclinical candidate studies.
MK-1468 is also being evaluated in a series of in vitro and in vivo genotoxicity studies, exploratory CV studies, and short-term dose-limiting toxicity (DLT) studies in rats and rhesus monkeys. These data support the progression of MK-1468 in GLP toxicology studies, the results of which will be reported at the appropriate time.
The target LRRK2 in the Synapse database
According to the statistics of the Synapse database, as of October 25, 2023, there are a total of 36 LRRK2 drugs worldwide, from 38 institutions, covering 14 indications, and 18 clinical trials are underway.
Summary
The current treatment of Parkinson's disease (PD) is mainly symptomatic, and specific analysis is required for different therapeutic targets. When treating PD with LRRK2 as the target, the main measures are to inhibit LRRK2 kinase activity or reduce LRRK2 expression. However, the multiple pathogenic factors of PD often interact and link together to cause the disease, complicating the related research. Therefore, research on the mechanism and treatment of PD should not be focused on a single target.
The development of PD treatment drugs needs to pay more attention to multi-pathway, multi-target research, such as screening compounds that can both inhibit oxidative stress and protect mitochondrial function. Therefore, elucidating the pathogenesis of PD and researching how to stop the degeneration of substantia nigra dopaminergic neurons are crucial for the further advancement of PD treatment research.
George W. Merck, the founder of Merck, once famously said, "We should always remember: medicines are produced for the sake of humanity, not for the pursuit of profit. As long as we hold on to this belief, profit will inevitably follow." Perhaps it is this simple and humble value that has enabled a pharmaceutical company spanning five centuries to become a lasting legend.
Reference:
1.Solomon D. Kattar et.al;Discovery of MK-1468: A Potent, Kinome-Selective, Brain-Penetrant Amidoisoquinoline LRRK2 Inhibitor for the Potential Treatment of Parkinson’s Disease,https://doi.org/10.1021/acs.jmedchem.3c01486.
2.Keylor, M. H.et.al; Structure-Guided Discovery of Aminoquinazolines as Brain-Penetrant and Selective LRRK2 Inhibitors. J. Med. Chem. 2022, 65, 838−856.