Cemiplimab-RWLC: brief review of its R&D progress and the clinical result in 2023 ESMO
On 23 Oct 2023, the clinical results of Cemiplimab plus chemotherapy versus chemotherapy in non-small cell lung cancer with PD-L1 ≥1% was reported at the ESMO Congress.
Cemiplimab-RWLC's R&D Progress
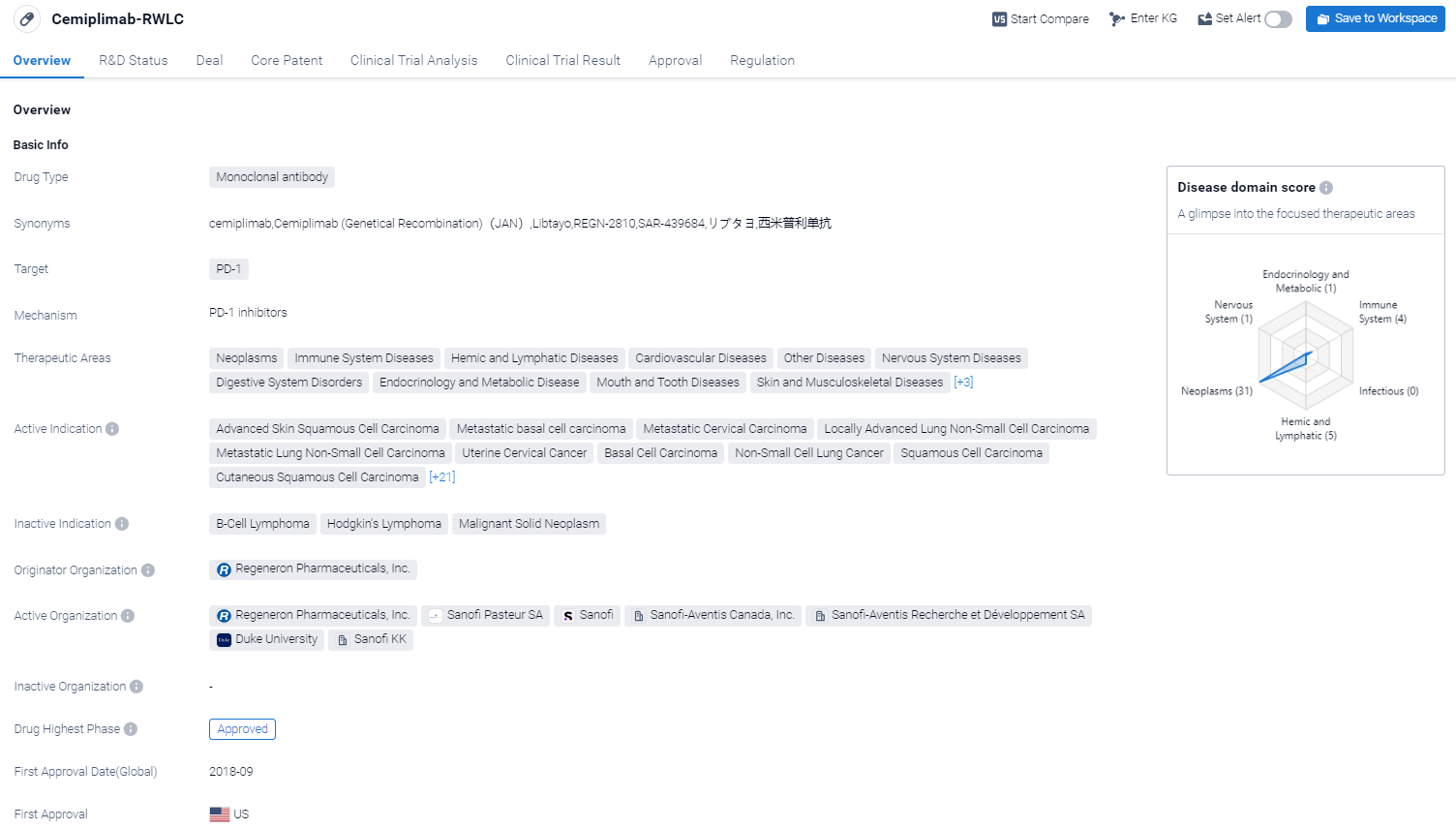
Cemiplimab-RWLC is a monoclonal antibody drug that targets PD-1, a protein involved in regulating the immune system. It has been approved for use in the treatment of various neoplasms, immune system diseases, hemic and lymphatic diseases, cardiovascular diseases, and other diseases.
According to the Patsnap Synapse, The drug was developed by Regeneron Pharmaceuticals, Inc. and received its first approval in the United States in September 2018. It has also reached Phase 3 of clinical trials in China. And the clinical trial areas for Cemiplimab-RWLC are primarily in the United States, China, and United Kingdom. The key indication is Neoplasms.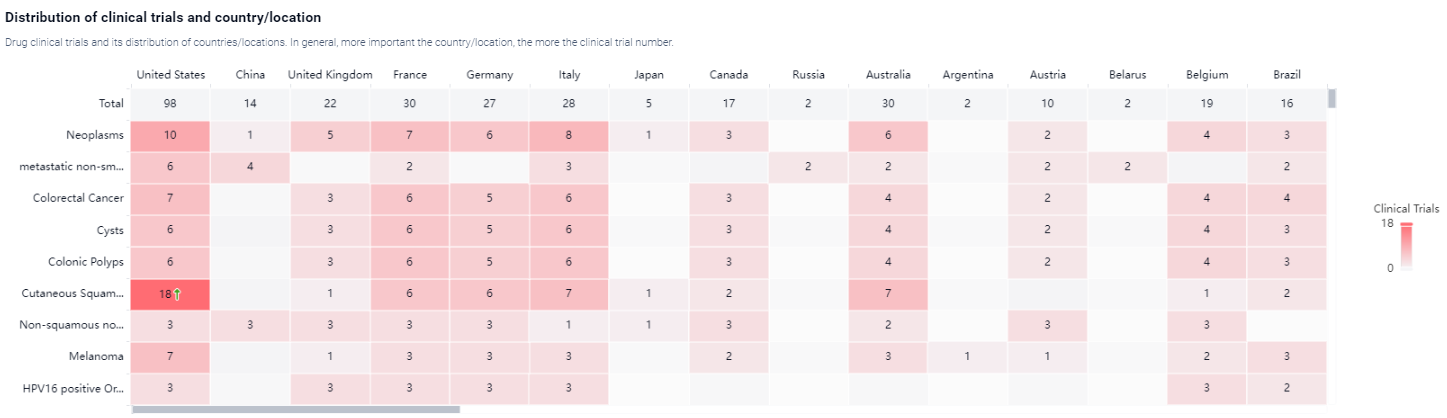
Detailed Clinical Result of Cemiplimab-RWLC
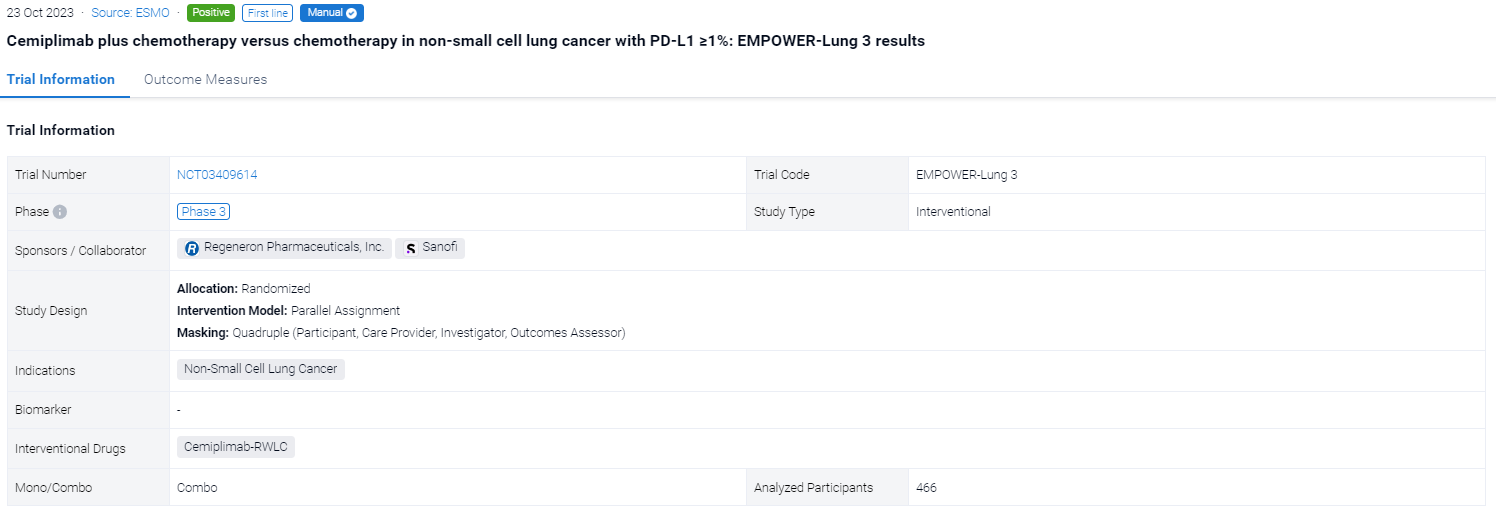
The randomized, parallel assignment, quadruple masking clinical trial (NCT03409614) was aimed to evacuate the efficacy of Cemiplimab plus chemotherapy versus chemotherapy in non-small cell lung cancer.
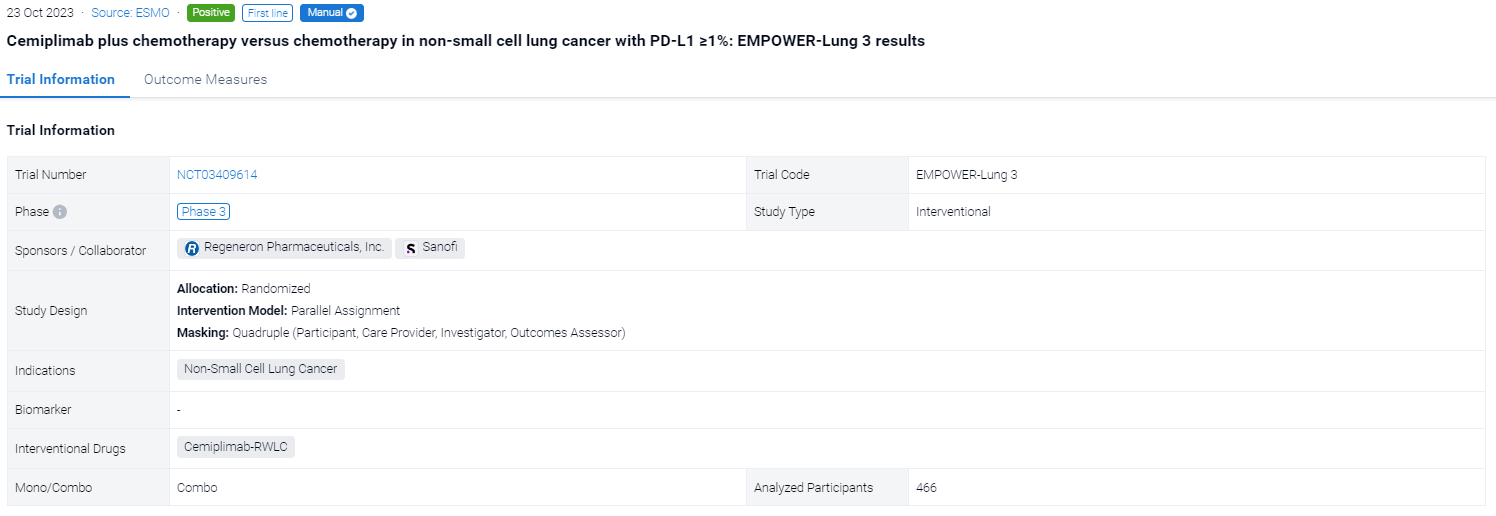
In this study, a total of 466 patients were randomised 2:1 to receive 4 cycles of platinum-doublet chemo in combination with cemiplimab 350 mg (n=312) or with placebo (n=154) every 3 weeks up to 108 weeks. The primary endpoint was OS. Secondary endpoints included PFS and ORR. This post hoc analysis included patients with PD-L1 ≥1% with ∼2-year follow-up data.
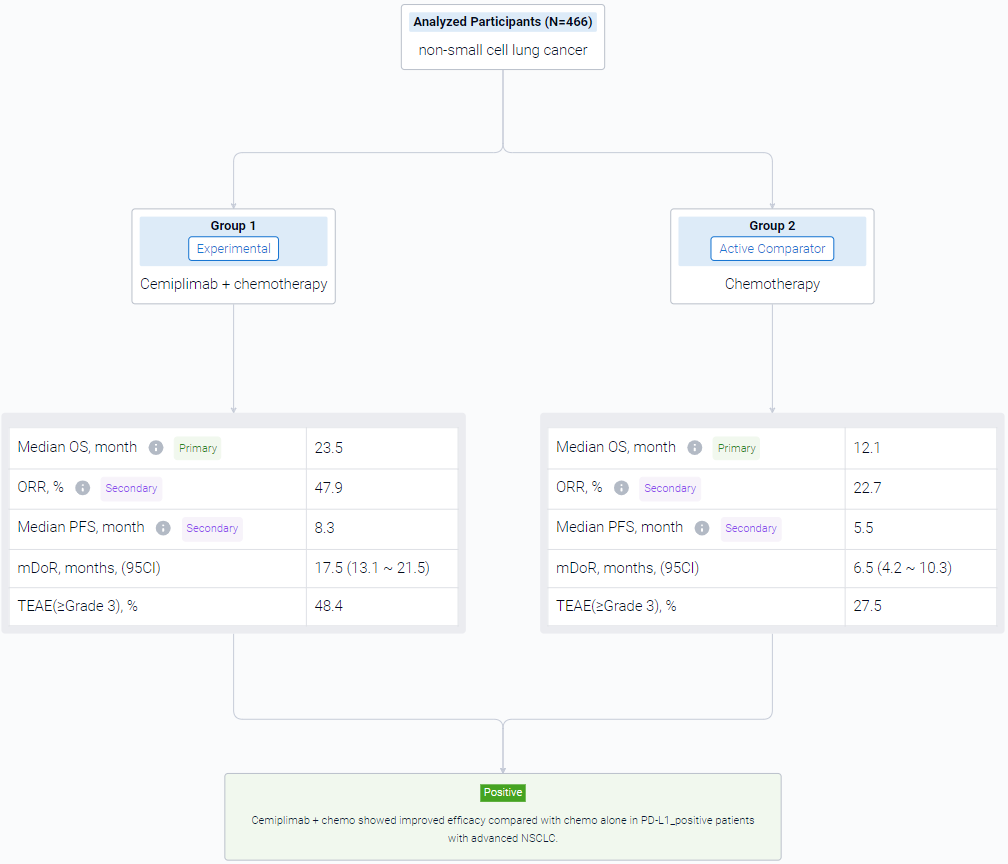
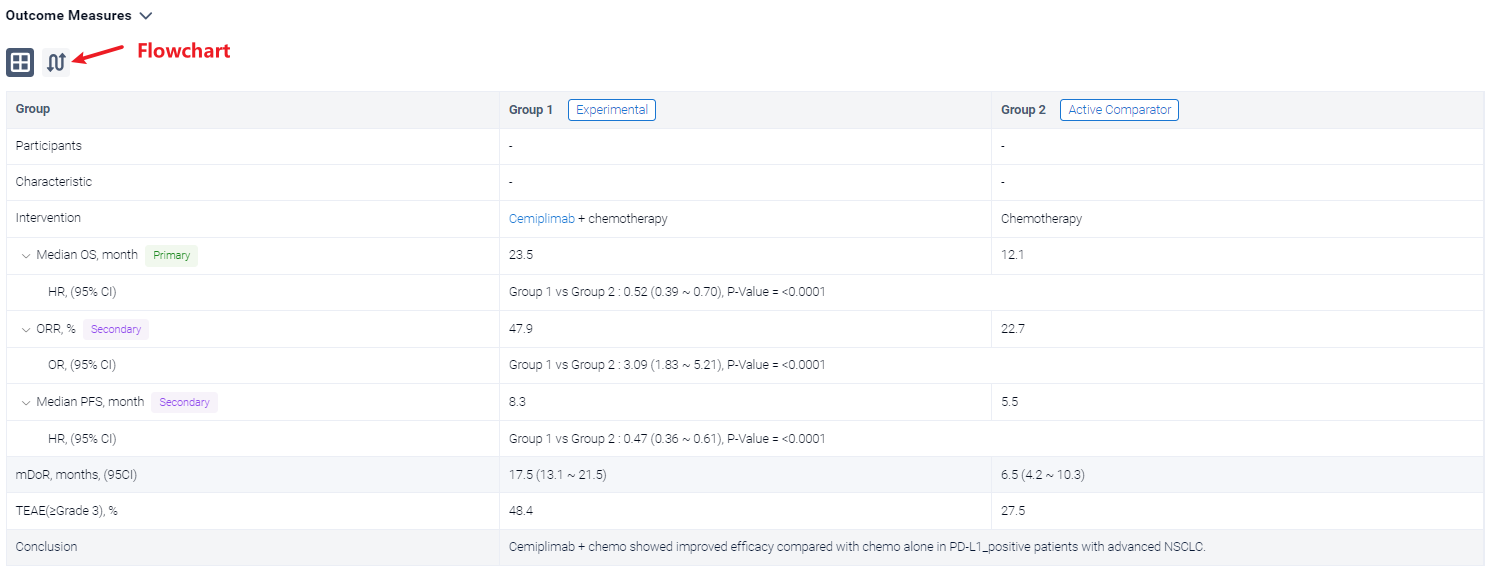
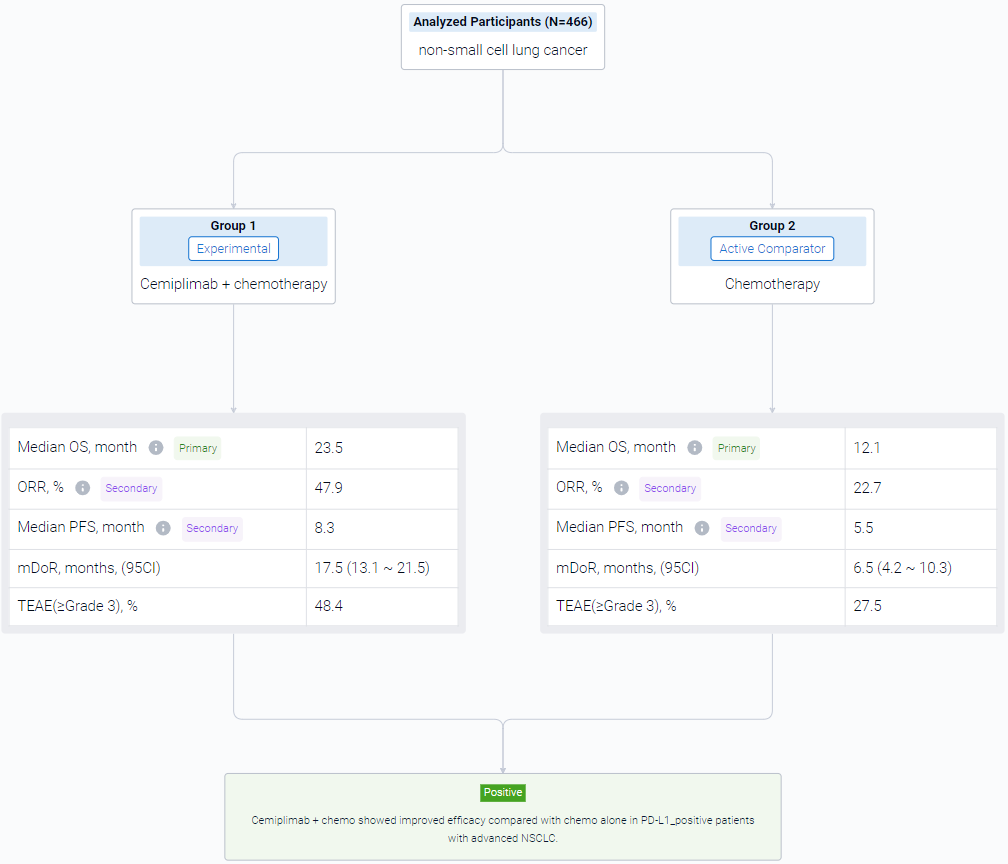
The result showed that In 327 patients with PD-L1 ≥1%, median OS was longer with cemiplimab + chemo than chemo alone (23.5 vs 12.1 months; HR: 0.52, P<0.0001). Median PFS was 8.3 months with cemiplimab + chemo versus 5.5 months with chemo alone (HR: 0.47, P<0.0001). ORRs were 47.9% vs 22.7%, with a response duration of 17.5 and 6.5 months. Grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 48.4% of patients with cemiplimab + chemo versus 27.5% with chemo alone. The safety profile for cemiplimab + chemo in patients with PD-L1 ≥1% was generally consistent with that in overall patients.
It can be concluded that Cemiplimab + chemo showed improved efficacy compared with chemo alone in PD-L1–positive patients with advanced NSCLC.
How to Easily View the Clinical Results Using Synapse Database?
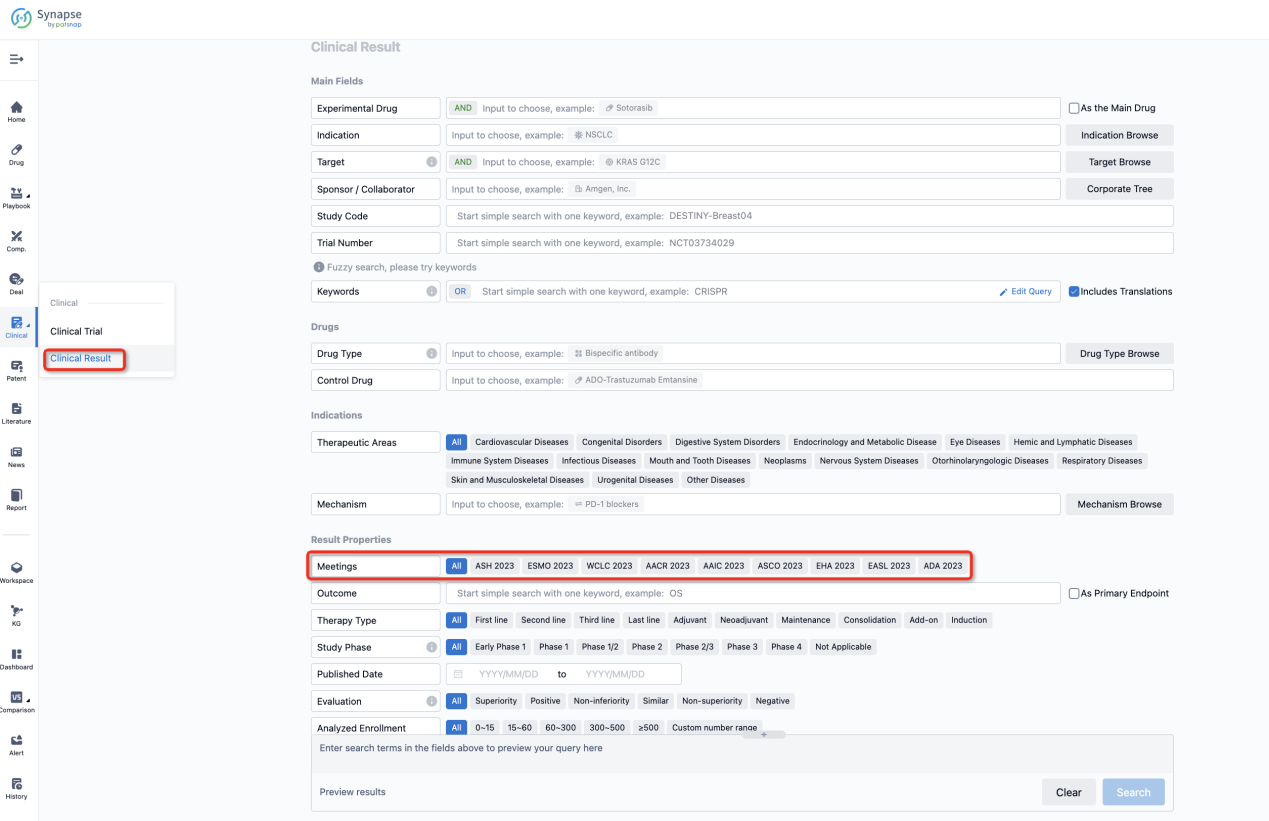
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
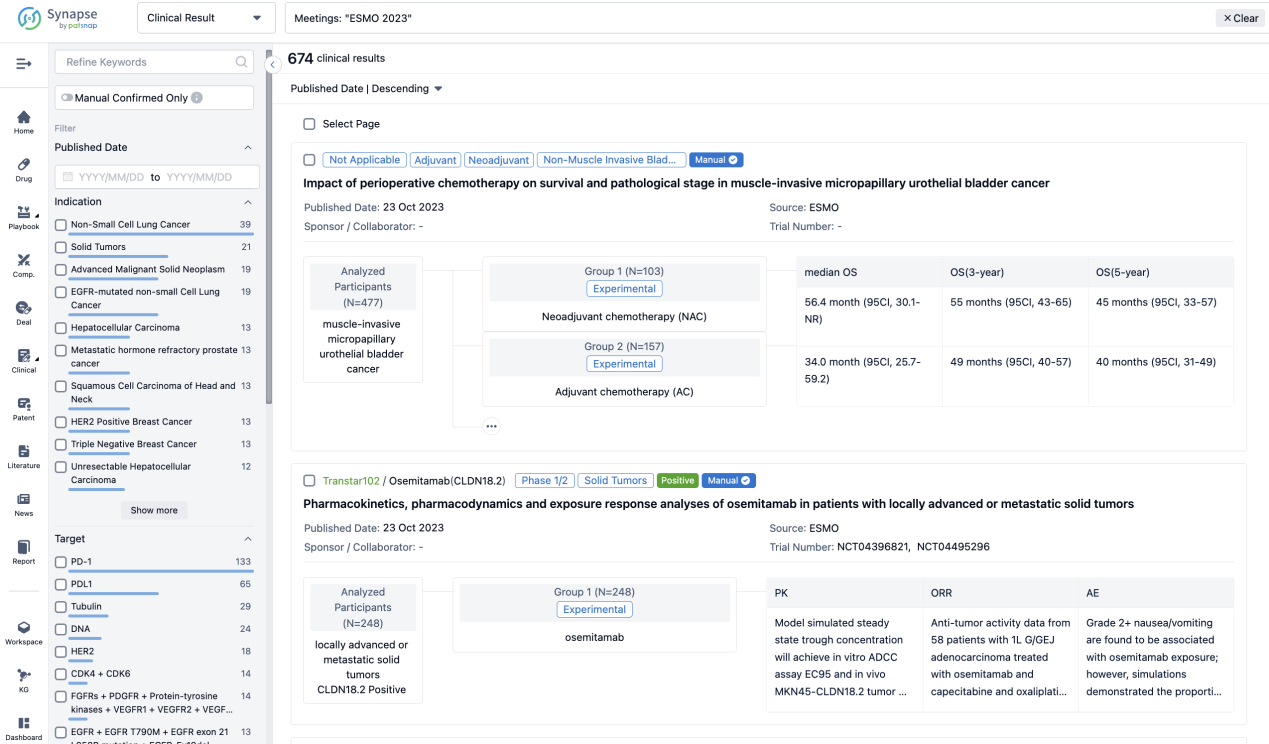
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
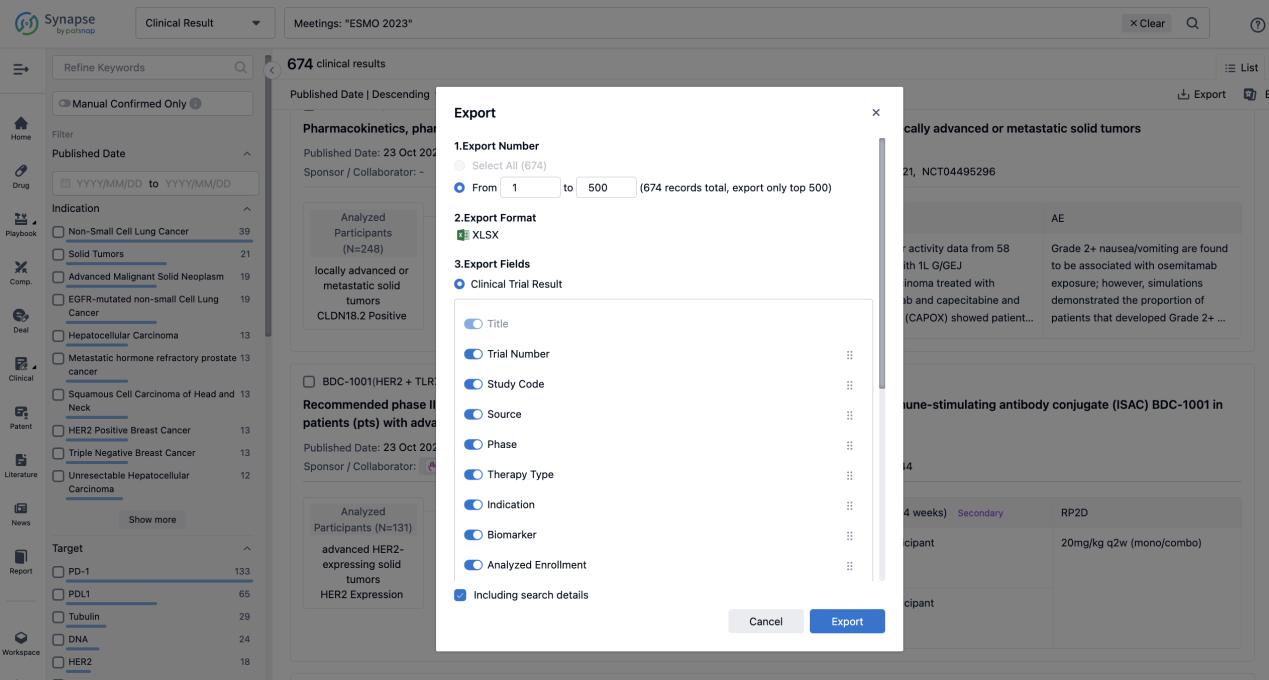
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!