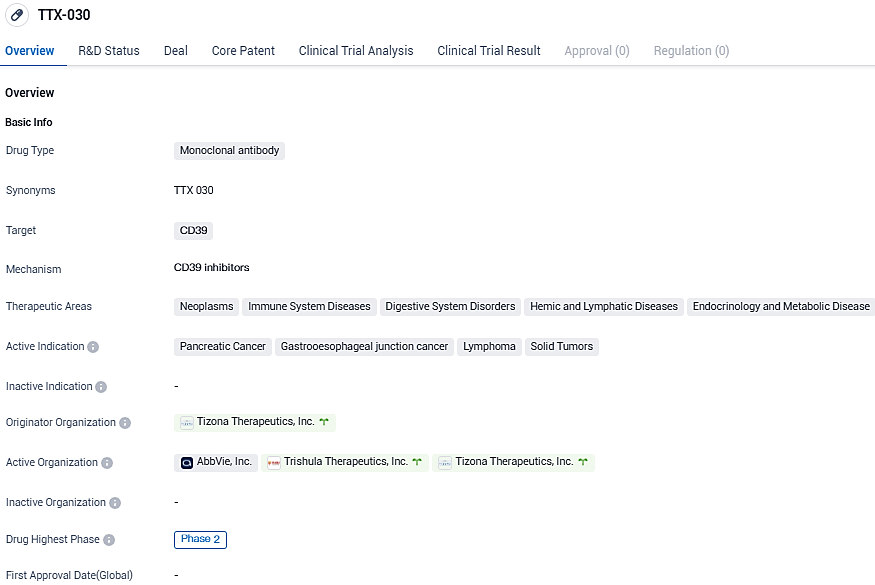
Trishula Therapeutics begins Phase 2 trial of TTX-030 for advanced pancreatic cancer
Trishula Therapeutics, Inc., a private biotech firm in the clinical phase, has declared the commencement of a Phase 2 trial that will focus on appraising the effectiveness and safety of TTX-030. This new trial involves an anti-CD39 antibody, which could be a premier in its class.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The effectiveness of TTX-030 in conjunction with chemotherapy, with budigalimab (a potential anti-PD-1 antibody) optionally included, will be examined against exclusively using chemotherapy as the primary therapy approach for patients suffering from metastatic pancreatic ductal adenocarcinoma.
"We urgently need fresh treatment strategies for pancreatic cancer," stated Anil Singhal, Chief Executive at Trishula Therapeutics. "Our aim is to build upon the encouraging outcomes from the Phase 1 trials with TTX-030 so as to progressively enhance the treatment realm, potentially benefiting patients struggling with advanced pancreatic cancer." The plan is for the research to involve roughly 180 patients worldwide.
Patients will be assigned to one of three cohorts, each receiving different combinations of TTX-030 and chemotherapy, TTX-030 plus chemotherapy and budigalimab, or exclusively chemotherapy. The trial's main goal is PFS in a biomarker-enriched population, with secondary objectives including PFS in the entire population, safety, objective response rate, response duration, and overall survival.
Trishula will carry on the development of TTX-030 with AbbVie Inc.
According to the conditions of the partnership agreement, AbbVie will be endowed with the option to license TTX-030 for future advancements upon the completion of this Phase 2 study. TTX-030, a likely first-in-class, anti-CD39 antibody, is engineered to restrict the activity of CD39, which is an enzyme catalyzing the conversion of adenosine triphosphate into adenosine monophosphate – the first stage in the production of adenosine in the tumor microenvironment.
This conversion is prevented by TTX-030, ensuring the maintenance of high extracellular ATP levels that stimulate immune activities, activating dendritic and myeloid-derived cells and fostering both inherent and adaptive anti-tumor immunity.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
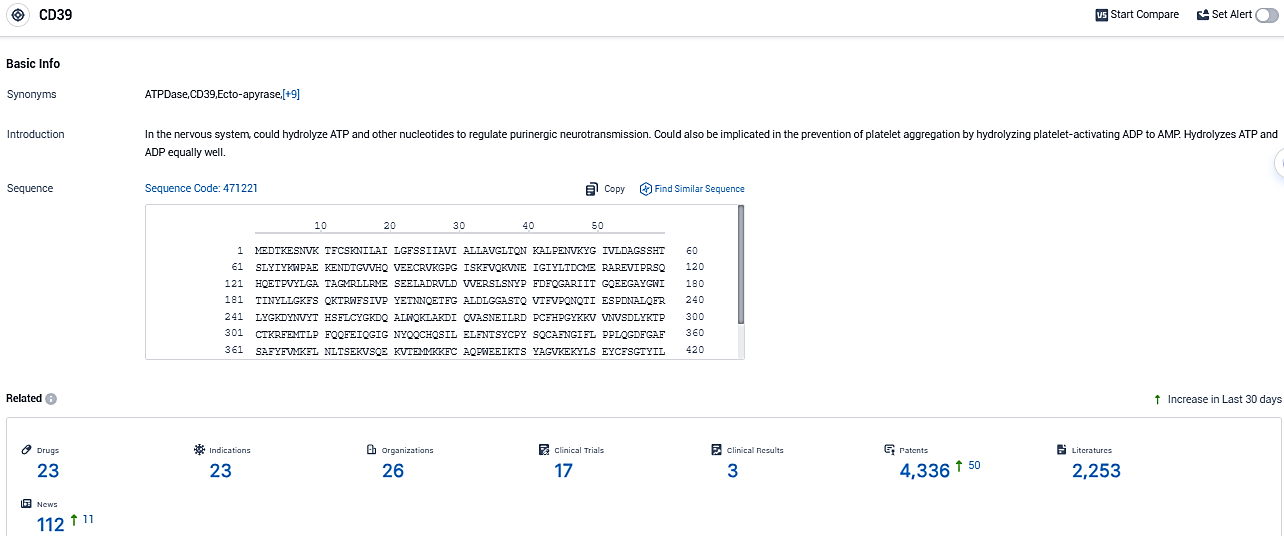
According to the data provided by the Synapse Database, As of November 16, 2023, there are 23 investigational drugs for the CD39 and integrin target, including 23 indications, 26 R&D institutions involved, with related clinical trials reaching 17, and as many as 4336 patents.
With active indications in pancreatic cancer, gastroesophageal junction cancer, lymphoma, and solid tumors, TTX-030 holds promise as a potential treatment option. However, further clinical trials and regulatory approval are still required to determine its safety and efficacy.