Cerevel Therapeutics Reveals Encouraging Initial Outcomes from Phase 3 Supplementary Study of Tavapadon in Parkinson’s Disease Patients
Cerevel Therapeutics, a firm focused on unlocking the secrets of the brain to address neurological disorders, disclosed encouraging primary outcomes from its critical Phase 3 TEMPO-3 trial evaluating tavapadon. Notably, tavapadon is the first and only D1/D5 receptor partial agonist explored as a daily therapy for Parkinson’s disease.
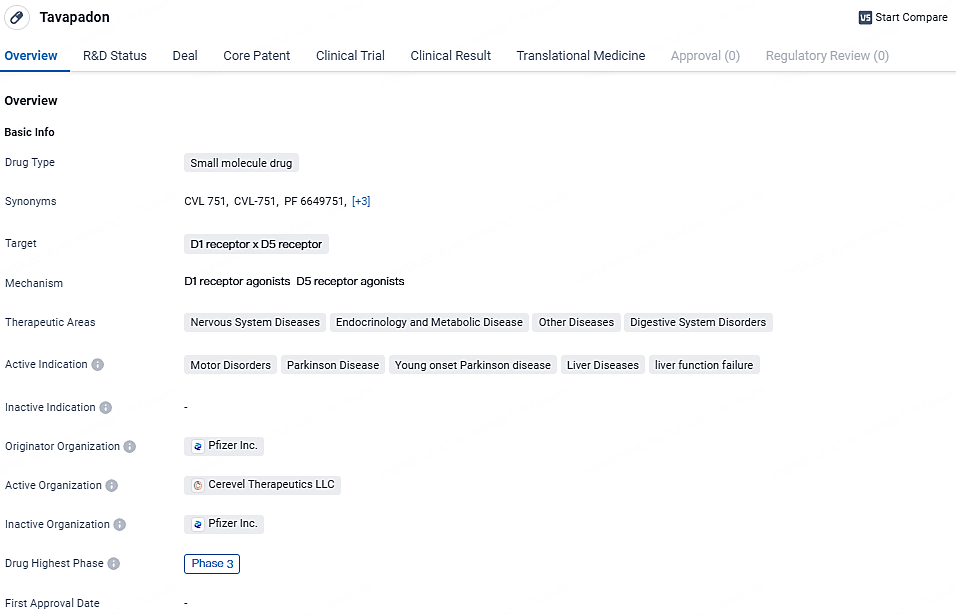
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The TEMPO-3 study assessed the effectiveness and safety of tavapadon when administered alongside levodopa in adult patients. The research achieved its primary goal – showing that patients receiving tavapadon in addition to levodopa noted a significant improvement with an increase of 1.1 hours in their total "on" time without severe dyskinesia compared to those who received levodopa with a placebo. Furthermore, a notable decrease in "off" time, which was a crucial secondary goal, was also achieved in the tavapadon group.
Raymond Sanchez, M.D., chief medical officer at Cerevel Therapeutics, stated, "The distinctive method by which tavapadon activates D1/D5 dopamine receptors shows great promise in offering individuals with Parkinson’s disease an optimal mix of motor function, safety, and comfort.”
Sanchez also noted the promising outcomes of the current study and expressed anticipation for upcoming disclosures later in the year from the stand-alone tavapadon trials, TEMPO-1 and TEMPO-2, aiming to further verify the drug’s advantages for individuals afflicted with Parkinson's disease.
The tolerance to tavapadon was broadly acceptable in the TEMPO-3 study, maintaining a safety profile similar to earlier studies of the drug. Most side effects were mild or moderate.
Hubert H. Fernandez, M.D., global principal investigator and the endowed James and Constance Brown chair in movement disorders at Cleveland Clinic, emphasized the urgent need for innovative treatments for Parkinson's disease, which is rapidly becoming the most prevalent neurodegenerative disorder globally. He highlighted the necessity for options that balance dopamine activity and provide sustained motor control with fewer burdensome side effects.
The complete findings of the TEMPO-3 trial are planned to be presented in future scientific meetings and will contribute to regulatory filings for tavapadon as a new therapeutic option for Parkinson’s disease. Initial results from the Phase 3 solo therapy trials, TEMPO-1 and TEMPO-2, are expected later in the second half of 2024.
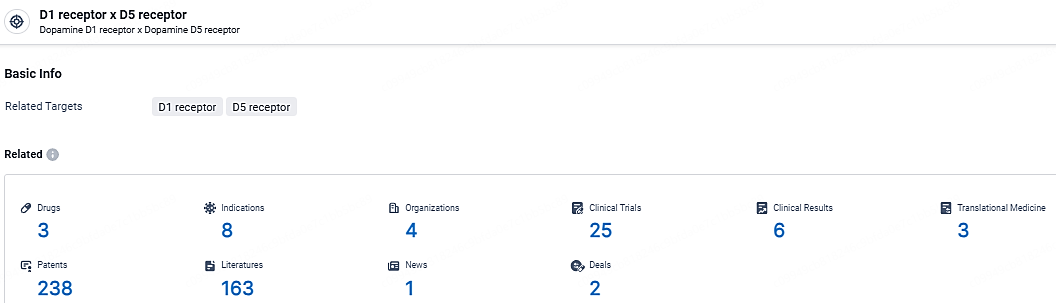
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 22, 2024, there are 3 investigational drugs for the D1/D5 receptor, including 8 indications, 4 R&D institutions involved, with related clinical trials reaching 25, and as many as 238 patents.
Tavapadon shows promise as a potential treatment option for various diseases within the nervous system, endocrinology, metabolic diseases, and digestive system disorders. Its advancement to Phase 3 indicates that it has demonstrated positive results in earlier stages of development. Further clinical trials will be necessary to establish its safety and efficacy before it can be considered for regulatory approval.