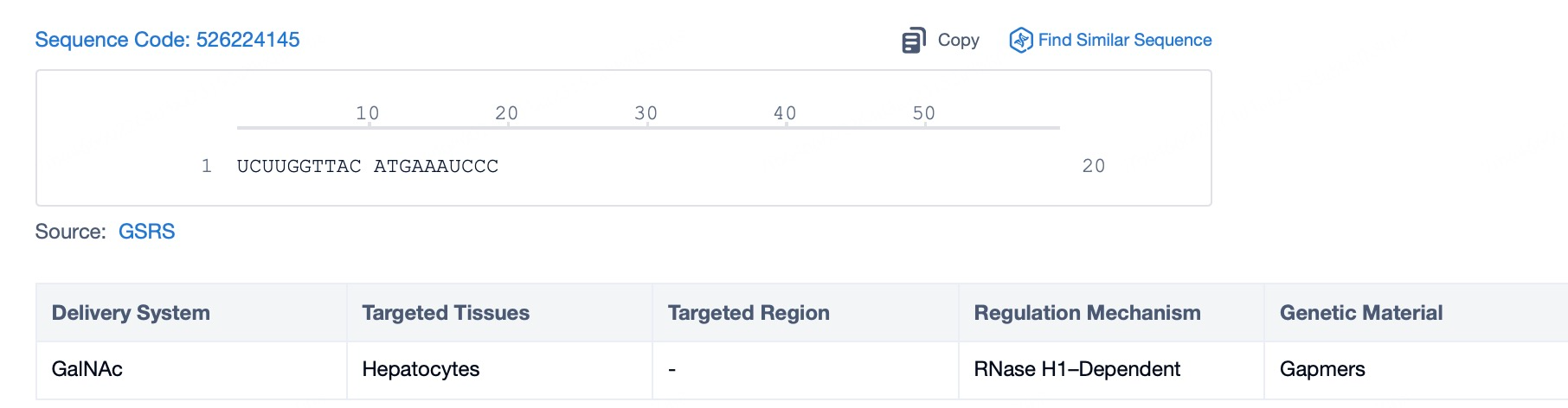
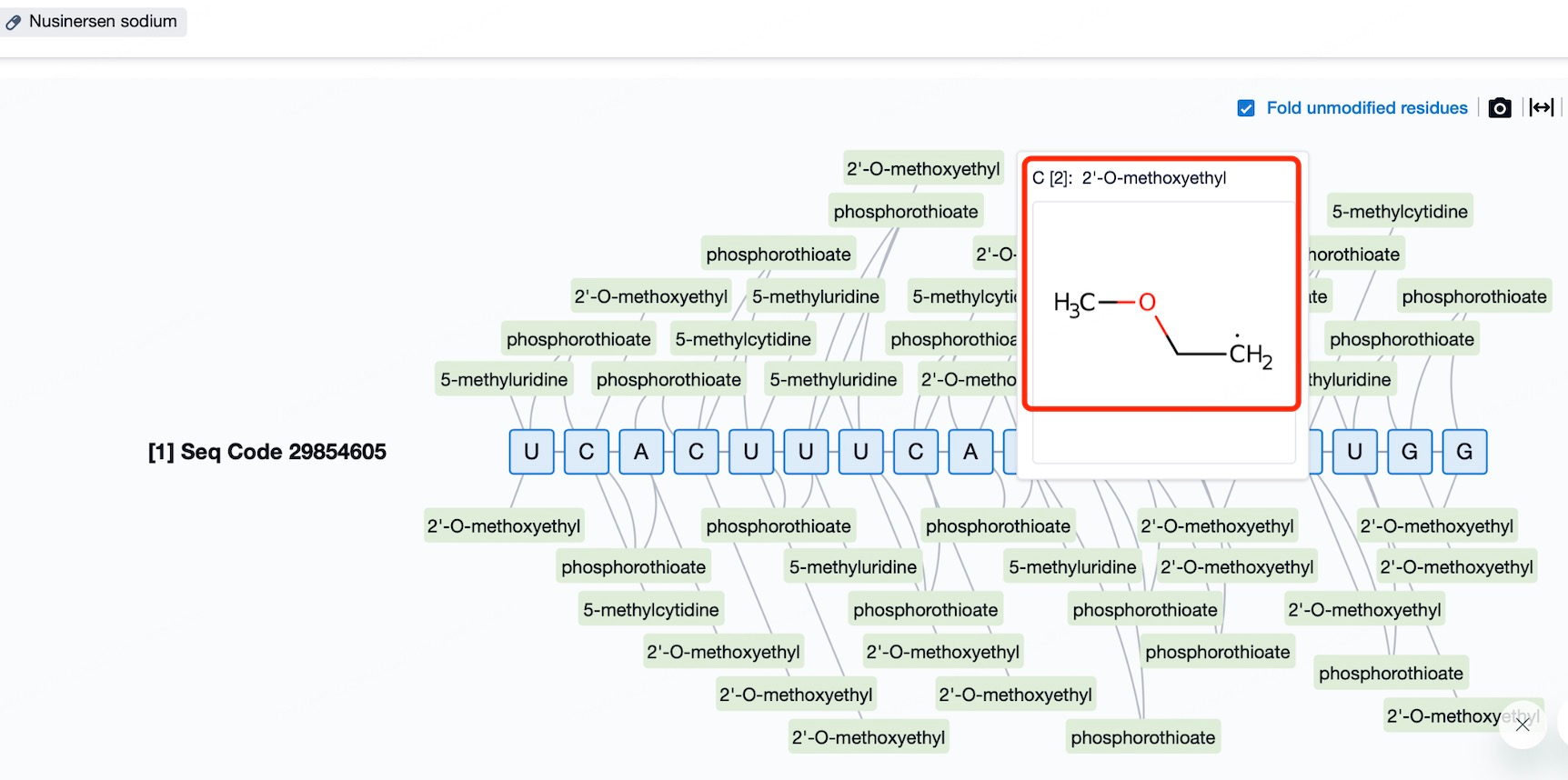
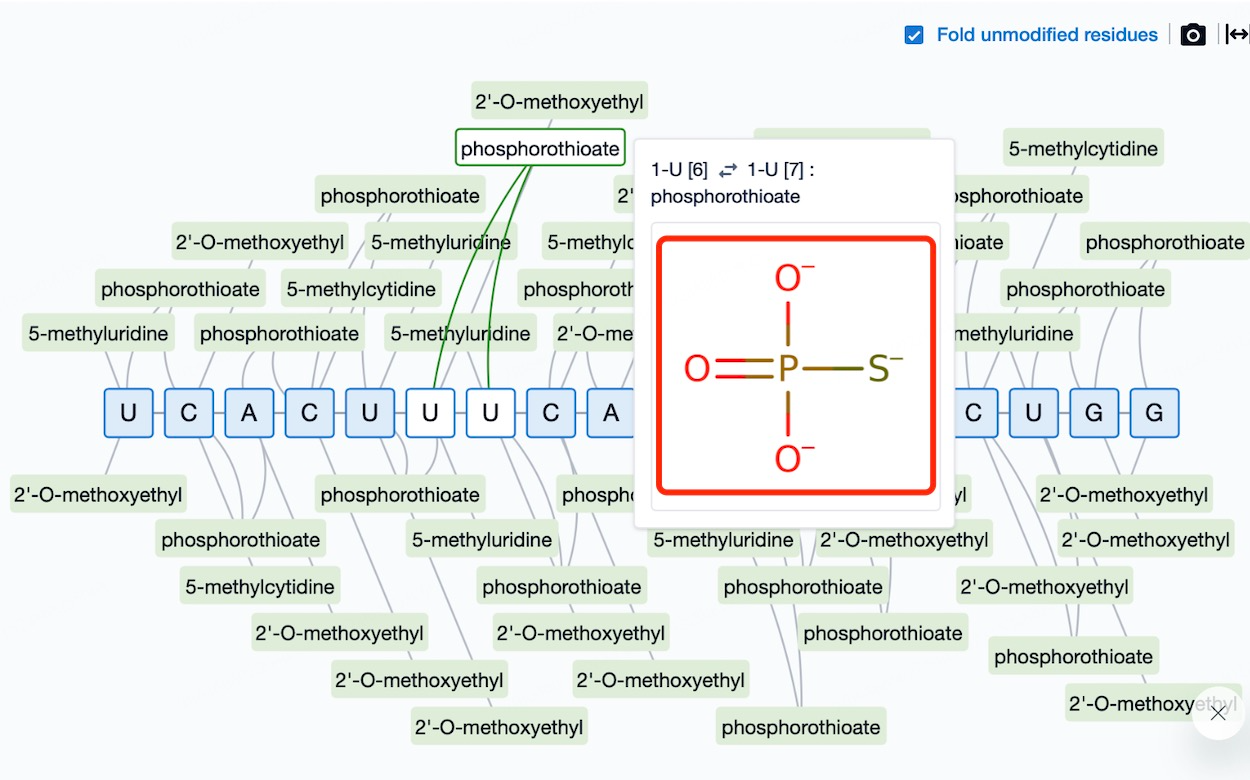
Chemical Modifications and Consequences: A Deep Dive into ASO Safety Profiles
With the rapid advancement of precision medicine, antisense oligonucleotide (ASO) drugs have emerged as a promising class of therapeutics capable of specifically modulating gene expression for the treatment of various genetic and acquired diseases. However, as these innovative therapies gain momentum, their potential safety concerns—particularly hepatotoxicity, nephrotoxicity, and thrombocytopenia—have drawn increasing attention. These adverse effects not only impact patient experience and prognosis but also pose new challenges for clinical application. Furthermore, the immunogenicity of ASOs remains a critical issue, as their interactions with the immune system can trigger complex physiological responses. Understanding the mechanisms underlying these safety concerns and developing effective mitigation strategies is essential for advancing ASO drug development and safeguarding patient health.
Hepatotoxicity of ASO Drugs
Hepatotoxicity is one of the most frequently observed adverse effects of ASO therapies, and its severity and mechanisms are influenced by various factors, particularly the type of chemical modification employed. Existing studies suggest that liver toxicity is largely determined by the chemical modification patterns of ASOs, alterations to the nucleotide backbone, and the resulting interactions with intracellular proteins.
Different chemical modifications contribute differently to hepatotoxicity. Low-affinity modifications such as 2'-O-methyl (2'OMe) and 2'-O-methoxyethyl (MOE) typically require higher doses to achieve therapeutic tissue concentrations, which can lead to toxicity in preclinical species. However, in mammalian systems, even medium-affinity (MOE) and high-affinity modifications like locked nucleic acids (LNAs) have been associated with liver or kidney toxicity. While higher affinity theoretically allows for lower dosing, it may simultaneously elevate the risk of organ-specific toxicity, including hepatotoxicity.
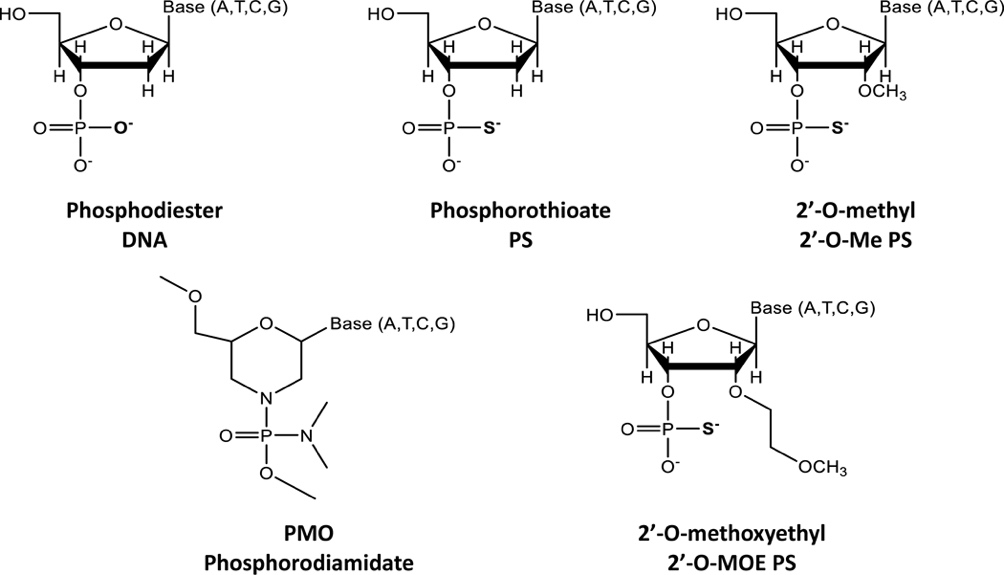
Phosphodiester (P=O) linkages within ASO molecules may significantly increase liver toxicity. Unmodified DNA/RNA duplexes are typically susceptible to nuclease degradation, and while phosphorothioate (PS) modifications improve stability, they also alter interactions with cellular components, potentially leading to off-target biological effects. Furthermore, PS modifications have been implicated in inducing thrombocytopenia, likely due to increased binding to platelet surface receptors.
To minimize liver toxicity, researchers are investigating multiple strategies, including optimizing chemical modification schemes, improving delivery systems to enhance target specificity, and selecting safer and more predictive animal models for early toxicology assessments. Given inter-individual variability, the development of predictive models to identify patient populations at higher risk of ASO-induced liver injury is also crucial. These models would support the creation of personalized treatment plans aimed at minimizing toxicity while preserving therapeutic efficacy.
Nephrotoxicity of ASO Drugs
The kidneys, as the primary excretory organs, filter metabolic waste and excess fluids from the bloodstream for elimination via urine. Any compound that enters systemic circulation, including ASOs, is processed through the kidneys. Due to their relatively large molecular size, ASOs can pass through the glomerular filtration barrier and accumulate in renal tubular epithelial cells, where they may induce direct cytotoxic effects or disrupt cellular function. Given the kidneys' extensive blood supply, they are particularly vulnerable to drug-induced toxicity.
Initially, liver and kidney toxicities were thought to result mainly from drug accumulation. However, research has shown that even at lower doses, certain chemical modifications can cause significant kidney damage. These modifications may alter the physicochemical properties of ASOs, enhancing renal uptake and accumulation. They can also interfere with membrane receptors or intracellular components, triggering inflammatory responses or apoptosis.
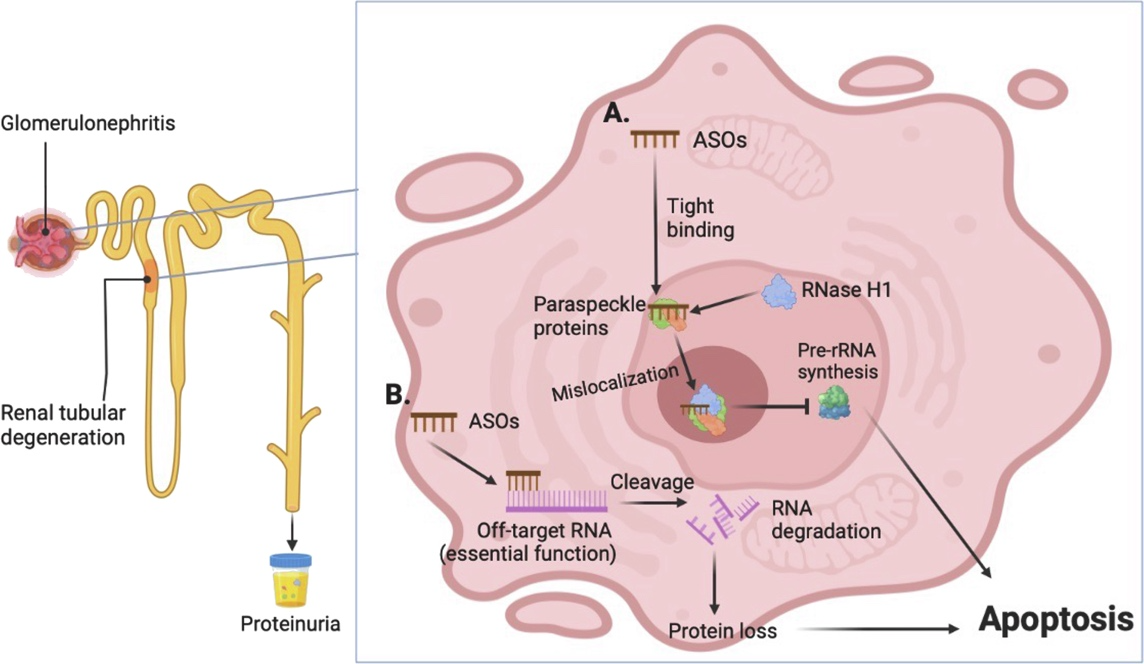
Recent studies have proposed several mechanisms underlying ASO-induced nephrotoxicity. Some modifications may alter the molecule’s charge, increasing its affinity for tubular epithelial cell surfaces. Others may disrupt normal signaling pathways within renal cells. Additionally, evidence suggests that ASOs may impair mitochondrial function, leading to disruptions in energy metabolism. Since mitochondria are essential for cellular energy production, dysfunction can result in cell injury or death. Understanding how ASOs affect mitochondrial integrity and developing strategies to mitigate such effects are vital steps in reducing renal toxicity. Epigenetic modifications may also play a role, as they influence gene expression and modulate cellular responses to ASO treatment. Elucidating these complex interactions is key to devising safer and more effective therapeutic approaches.
To address ASO-associated nephrotoxicity, researchers are exploring several mitigation strategies. Optimization of chemical modifications is a critical area—choosing functional groups that retain therapeutic activity while minimizing toxicity is essential for balancing efficacy and safety. Improving delivery systems is another priority, with targeted delivery technologies being developed to direct ASOs specifically to diseased tissues while avoiding off-target organ exposure. Finally, in clinical settings, close monitoring of renal function indicators and timely adjustments to dosing regimens are essential to prevent serious adverse events. By integrating these approaches, we can better manage the safety profile of ASO drugs and provide patients with more reliable and effective treatment options.
Thrombocytopenia Induced by ASO Drugs
Thrombocytopenia refers to a condition where the number of platelets in circulating blood falls below the normal range, typically defined as a platelet count of less than 150,000 per microliter. When platelet levels drop below approximately 50,000/µL, even minor injuries may cause bleeding. Further reduction to 10,000–20,000/µL can lead to spontaneous bleeding, even in the absence of obvious trauma. The causes of thrombocytopenia are varied and include, but are not limited to, insufficient platelet production in the bone marrow, autoimmune destruction of platelets, splenic sequestration, and drug-induced platelet destruction or inhibition.
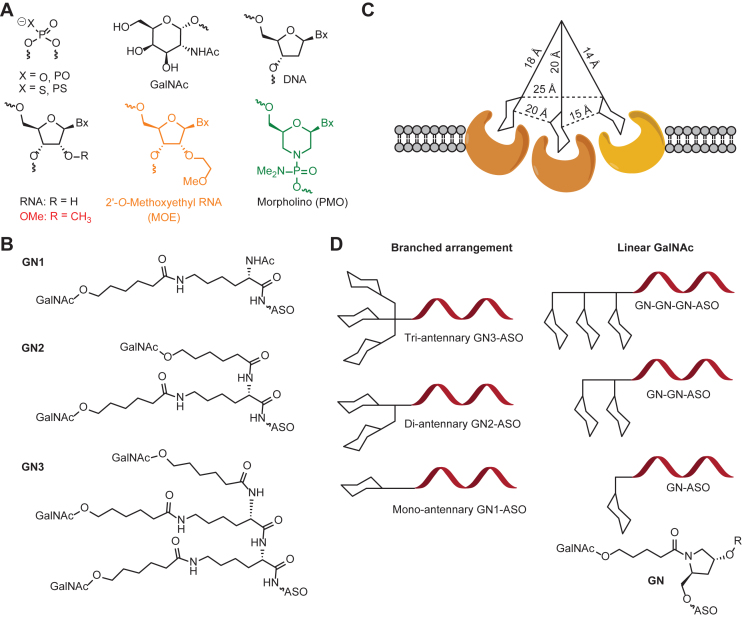
Notably, thrombocytopenia is a relatively common adverse effect observed with oligonucleotide drugs containing phosphodiester (P=O) or phosphorothioate (PS) backbone modifications. Studies have shown that PS-modified ASOs can specifically bind to platelet glycoprotein VI (GPVI), leading to platelet activation and the formation of platelet-leukocyte aggregates (PLAs). This phenomenon has been confirmed across multiple species and experimental models. Specifically, PS-ASO binding to GPVI activates platelets, prompting the release of granule contents and promoting interactions between platelets and other cells such as leukocytes. This process not only impairs platelet function but may also trigger inflammatory responses and increase bleeding risk in patients.
Furthermore, the mechanisms underlying thrombocytopenia are complex. In addition to factors associated with oligonucleotide drugs, other causes such as infections, malignancies, and autoimmune diseases can also contribute. For example, some viral infections can directly damage megakaryocytes in the bone marrow, thereby reducing platelet production. In cancer patients, malignant cells may infiltrate the bone marrow and disrupt normal hematopoiesis. When thrombocytopenia is determined to be drug-induced, discontinuation or switching to alternative therapies may be warranted.
In clinical practice, managing thrombocytopenia requires a comprehensive assessment of its etiology and severity to develop personalized treatment plans. If ASOs, such as PS-ASOs, are the cause, dose adjustment or replacement therapy may be necessary. Close monitoring of platelet levels and appropriate supportive care, such as platelet transfusion, are essential to ensure patient safety and control disease progression. Understanding and managing thrombocytopenia is particularly critical in the context of emerging therapies such as oligonucleotide drugs.
Immunogenicity of ASO Drugs
The immunogenicity of antisense oligonucleotide (ASO) drugs is a complex and critical issue, as these agents share structural similarities with naturally occurring nucleic acids, which may elicit immune recognition and response. Although chemical modifications are routinely employed during drug development to enhance stability and reduce toxicity, certain modifications may paradoxically enhance the risk of immune activation. For instance, guanine (G)- or uracil (U)-rich sequences are more likely to bind to Toll-like receptors 7 and 8 (TLR7/8), thus activating the innate immune system.
When ASOs contain sequences recognizable by pattern recognition receptors (PRRs), particularly TLRs, they may trigger immune responses. TLRs are essential PRRs responsible for detecting pathogen-associated molecular patterns (PAMPs) and initiating host defense mechanisms during infection. In humans, TLR7 and TLR8 are expressed in plasmacytoid dendritic cells and monocytes/macrophages, respectively, and are capable of recognizing unmethylated CpG motifs in DNA or GU-rich regions in single-stranded RNA, thereby activating downstream signaling pathways that induce proinflammatory cytokines and other mediators.
Beyond direct TLR activation, ASOs may also influence the immune system through alternative pathways. For example, they may form complexes in vivo or exhibit cross-reactivity with self-antigens, potentially contributing to autoimmune reactions. Thus, it is imperative to carefully balance the efficacy and safety of ASO therapies during drug development. Researchers are actively exploring multiple strategies to minimize immunogenicity, including optimizing nucleotide composition, selecting appropriate chemical modifications, and enhancing delivery systems to ensure targeted delivery while minimizing off-target immune stimulation.
Conclusion
ASO drugs represent a novel and promising class of therapeutics in the era of precision medicine. Despite their potential, associated safety concerns—including hepatotoxicity, nephrotoxicity, thrombocytopenia, and immunogenicity—remain significant risk factors. Research has demonstrated that the type of chemical modification plays a decisive role in these adverse effects. While certain modifications enhance drug stability and targeting efficiency, they may also increase the risk of toxicity to vital organs or trigger unwanted immune responses.
To minimize these risks, scientists are working to optimize chemical modification strategies, improve delivery technologies for greater targeting precision, and develop predictive models that account for individual variability. In clinical settings, close monitoring of patients’ physiological parameters and timely adjustments to treatment regimens are essential for ensuring both safety and therapeutic efficacy. Together, these approaches aim to support the continued development of ASO drugs while safeguarding patient health.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Refrence
- 1.Wu H, Wahane A, Alhamadani F, Zhang K, Parikh R, Lee S, McCabe EM, Rasmussen TP, Bahal R, Zhong XB, Manautou JE. Nephrotoxicity of marketed antisense oligonucleotide drugs. Curr Opin Toxicol. 2022 Dec;32:100373. doi: 10.1016/j.cotox.2022.100373. Epub 2022 Oct 21.
- 2.Schmidt K, Prakash TP, Donner AJ, Kinberger GA, Gaus HJ, Low A, Østergaard ME, Bell M, Swayze EE, Seth PP. Characterizing the effect of GalNAc and phosphorothioate backbone on binding of antisense oligonucleotides to the asialoglycoprotein receptor. Nucleic Acids Res. 2017 Mar 17.




