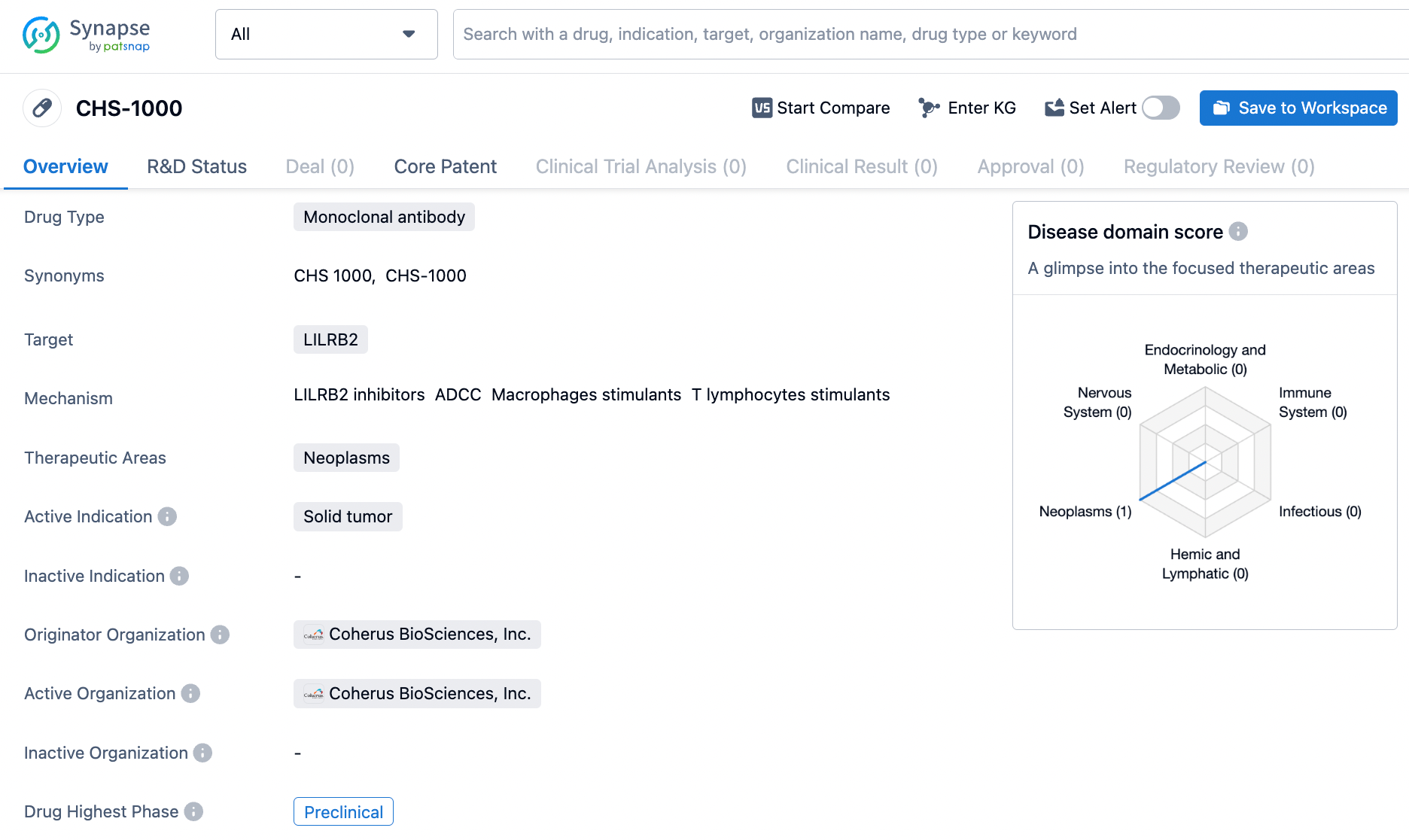
CHS-1000 - A Novel ILT4 Antagonist Unveiled at AACR 2024
Coherus BioSciences, Inc., unveiled research findings on their emerging immuno-oncology drug, CHS-1000, at the AACR Annual Meeting 2024 in San Diego, CA. This investigational agent is an innovative ILT4 monoclonal antibody. The shared evidence highlighted the robust efficacy of CHS-1000 in its specific attraction and high-affinity binding to the ILT4 receptor, known as LILRB2, blocking its interaction with corresponding ligands. This blockade lifts the suppression on the immune system, consequently triggering the activity of both human dendritic cells and T cells. Additionally, it encourages the shift of macrophages toward a pro-inflammatory M1 profile.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Immune evasion and PD-1 blockade resistance in cancer are strongly influenced by suppressive actions of myeloid cells in the cancerous ecological niche. The evidence provided by the research poster suggests that CHS-1000 could be effective in counteracting myeloid-driven suppression and promoting a pro-inflammatory immune response. "Modifying myeloid cells within the cancer niche is an innovative strategy for immuno-oncological treatments aimed at bypassing I-O therapeutic resistance, potentially extending benefits to a broader patient population affected by cancer," remarked Theresa LaVallee, Ph.D., the Head of Development at Coherus.
Dr. LaVallee added, “As our proprietary lead compound, the progress of CHS-1000 is particularly exhilarating for us, especially as we anticipate submitting the IND within the current quarter. Our strategy includes evaluating CHS-1000's effectiveness both as a monotherapy and when paired with LOQTORZI.”
Coherus BioSciences has pioneered the creation of CHS-1000, an innovative Fc-enhanced humanized IgG1 monoclonal antibody, intricately engineered to aim at ILT4 (LILRB2) to dismantle myeloid cell-induced immunosuppression in cancerous environments.
In the realm of preclinical research, CHS-1000 has been shown to induce a switch in the polarization of M2 macrophages toward an M1 pro-inflammatory state, along with bolstering the activation of both dendritic cells and T cells in laboratory settings. Looking ahead, Coherus BioSciences plans to submit a request for an investigational new drug (IND) in the second quarter of 2024, aiming to move CHS-1000 into human clinical trials later within the same year.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
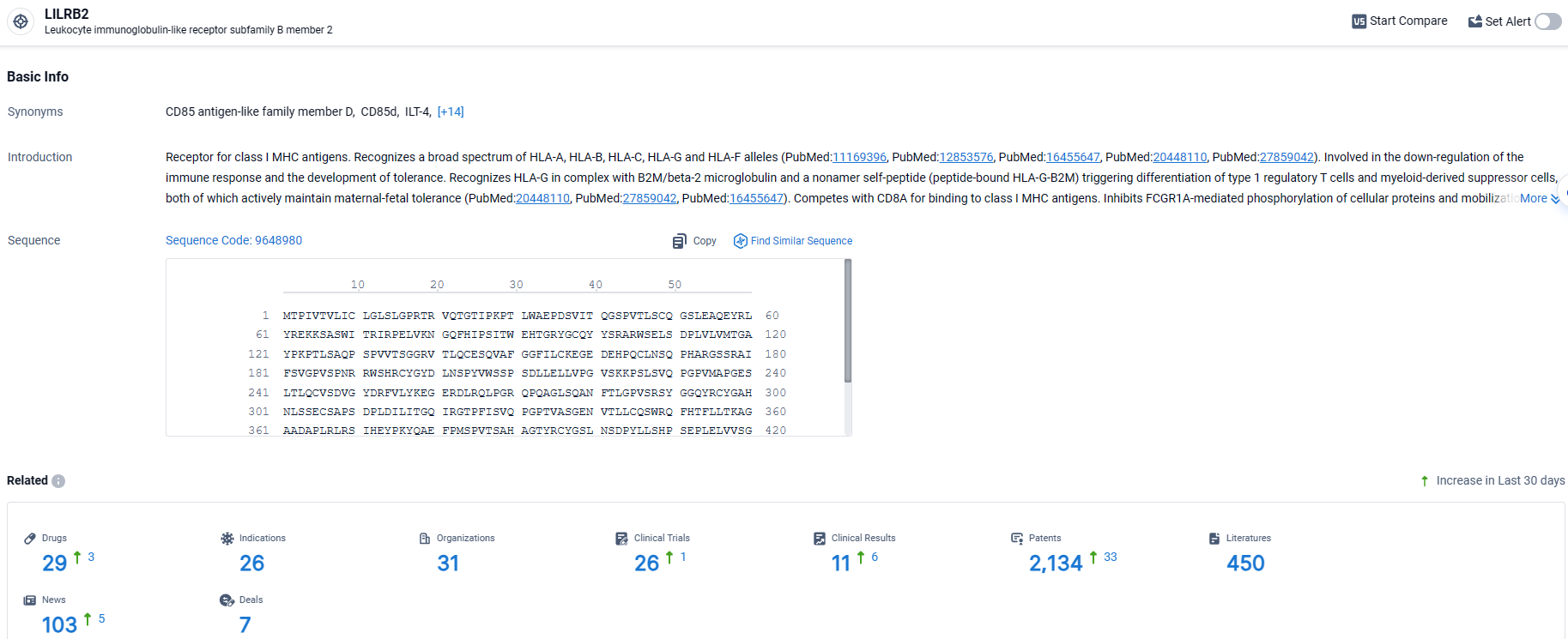
According to the data provided by the Synapse Database, As of April 10, 2024, there are 29 investigational drugs for the LILRB2 target, including 26 indications, 31 R&D institutions involved, with related clinical trials reaching 26, and as many as 2134 patents.
CHS-1000 is a monoclonal antibody drug being developed by Coherus BioSciences, Inc. for the treatment of solid tumors. However, since it is still in the preclinical phase, further research and testing are needed to determine its potential as a therapeutic option for patients with neoplasms.