Cidara Therapeutics has announced that Janssen has elected to move forward under their license agreement for novel drug-Fc conjugates targeting influenza
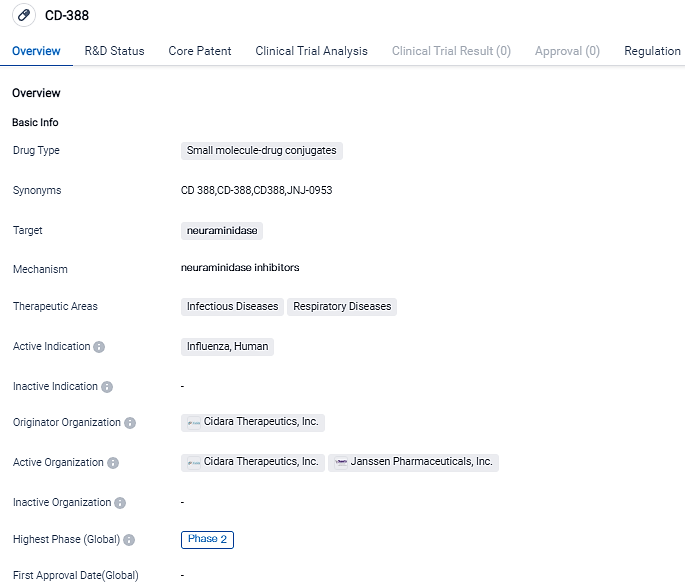
Cidara Therapeutics, Inc., a biotech company utilizing the proprietary Cloudbreak® platform to develop drug-Fc conjugate immunotherapies for serious diseases, has announced that Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, has provided Cidara with an Election to Proceed Notice for CD388 (JNJ-0953). CD388 is being developed as a universal prevention solution for both influenza A and B.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Janssen will assume responsibility for the future development, manufacturing, and commercialization of CD388, with the intention to transfer its rights and obligations under the agreement to another entity.
Jeffrey Stein, Ph.D., President and CEO of Cidara, stated, "The Election to Proceed notice is a significant achievement for CD388, our most advanced Cloudbreak® DFC program. The clinical and nonclinical data support the potential of a single dose to offer universal protection against seasonal and pandemic influenza strains A and B."
This milestone payment, along with potential future milestone payments, will provide Cidara with additional non-dilutive capital to advance our other Cloudbreak® DFC programs.
Within the scope of the exclusive worldwide collaboration with Janssen, Cidara is conducting Phase 1 and 2a clinical trials to assess the safety, pharmacokinetics, and pre-exposure prophylaxis efficacy of CD388 against influenza virus in healthy volunteers during a human challenge study.
Earlier this year, Cidara released promising interim efficacy and safety data from the Phase 2a study, demonstrating the reduction in viral replication in the respiratory tract and lower influenza incidence rate with a single dose of CD388 compared to placebo.
The treatment with CD388 was generally well-tolerated, with no report of treatment emergent adverse events leading to study discontinuation or serious adverse events. Cidara continues to collaborate with Janssen to complete the Phase 1 and Phase 2a trials, with Janssen providing reimbursement for all ongoing development activities as stipulated in the Janssen Collaboration Agreement. The complete CD388 Phase 2a data package is scheduled to be delivered to Janssen later this year.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
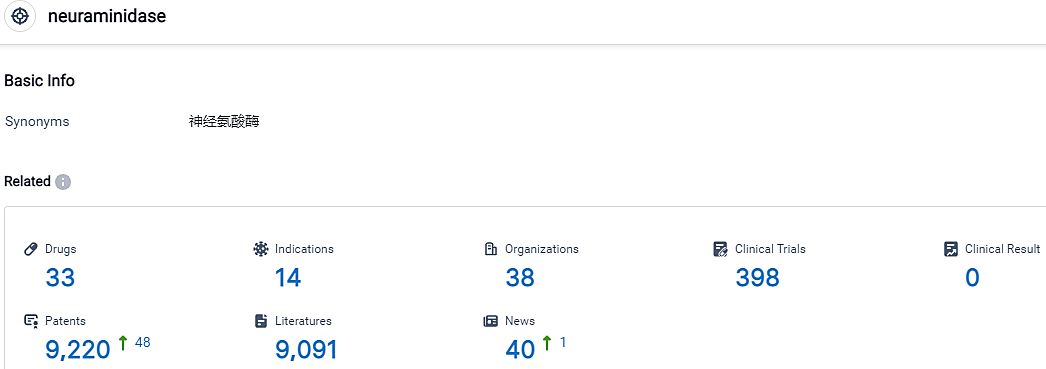
According to the data provided by the Synapse Database, As of September 11, 2023, there are 33 investigational drugs for the neuraminidase target, including 14 applicable indications,38 R&D institutions involved, with related clinical trials reaching 398,and as many as 9220 patents.
The current competitive landscape for the Influenza, Human indication is marked by ongoing research and development endeavors from numerous companies and countries. The future development in this area is expected to center around novel drug categories, including biosimilars, as well as advancements in preventive measures, such as vaccines.