BTK inhibitors, a field with promising prospects
BTK(Bruton's tyrosine kinase), is a non-receptor tyrosine kinase of the Tec family that is expressed in B cells, neutrophils, basophils, and platelets. It is also a key kinase in the B-cell receptor (BCR) signaling pathway and is expressed in all hematopoietic cells except T cells and terminally differentiated plasma cells.
BTK inhibitors can regulate the functions of various immune cells that are crucial to innate and adaptive immune responses. They are widely expressed in various types of malignant hematopoietic diseases and participate in the proliferation, differentiation, and apoptosis of B cells. Because of the high specificity of BTK small molecule inhibitors, they have significant advantages in treating B-cell malignancies and some B-cell immune diseases. BTK inhibitors have thus become one of the most promising drugs on the market for hematopoietic tumors.
BTK Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 169 BTK drugs worldwide, from 194 organizations, covering 116 indications, and conducting 1243 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.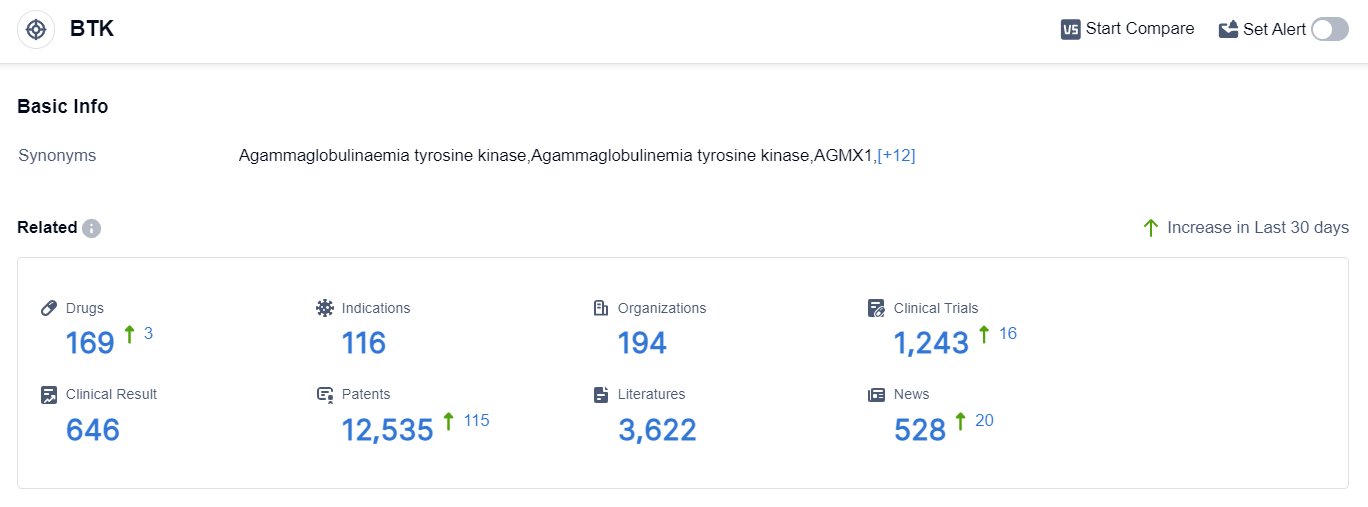 The current competitive landscape of target BTK is characterized by the presence of multiple companies actively developing drugs in various stages of development. AbbVie, Inc., Eli Lilly & Co., Johnson & Johnson, Sanofi, and Roche Holding AG are some of the key players with drugs in advanced stages of development.
The current competitive landscape of target BTK is characterized by the presence of multiple companies actively developing drugs in various stages of development. AbbVie, Inc., Eli Lilly & Co., Johnson & Johnson, Sanofi, and Roche Holding AG are some of the key players with drugs in advanced stages of development.
The approved indications for drugs targeting BTK include B-Cell Chronic Lymphocytic Leukemia, Mantle-Cell Lymphoma, Small Lymphocytic Lymphoma, Waldenstrom Macroglobulinemia, Marginal Zone B-Cell Lymphoma, and others.
Small molecule drugs and PROTACs are the most rapidly progressing drug types, indicating intense competition in the market. China and the United States are leading in terms of drug development under the target BTK, with China showing significant progress.
Overall, the future development of target BTK holds promise with a strong pipeline of drugs and active research and development efforts by various companies and countries/locations.
First Generation BTK Inhibitors:Ibrutinib
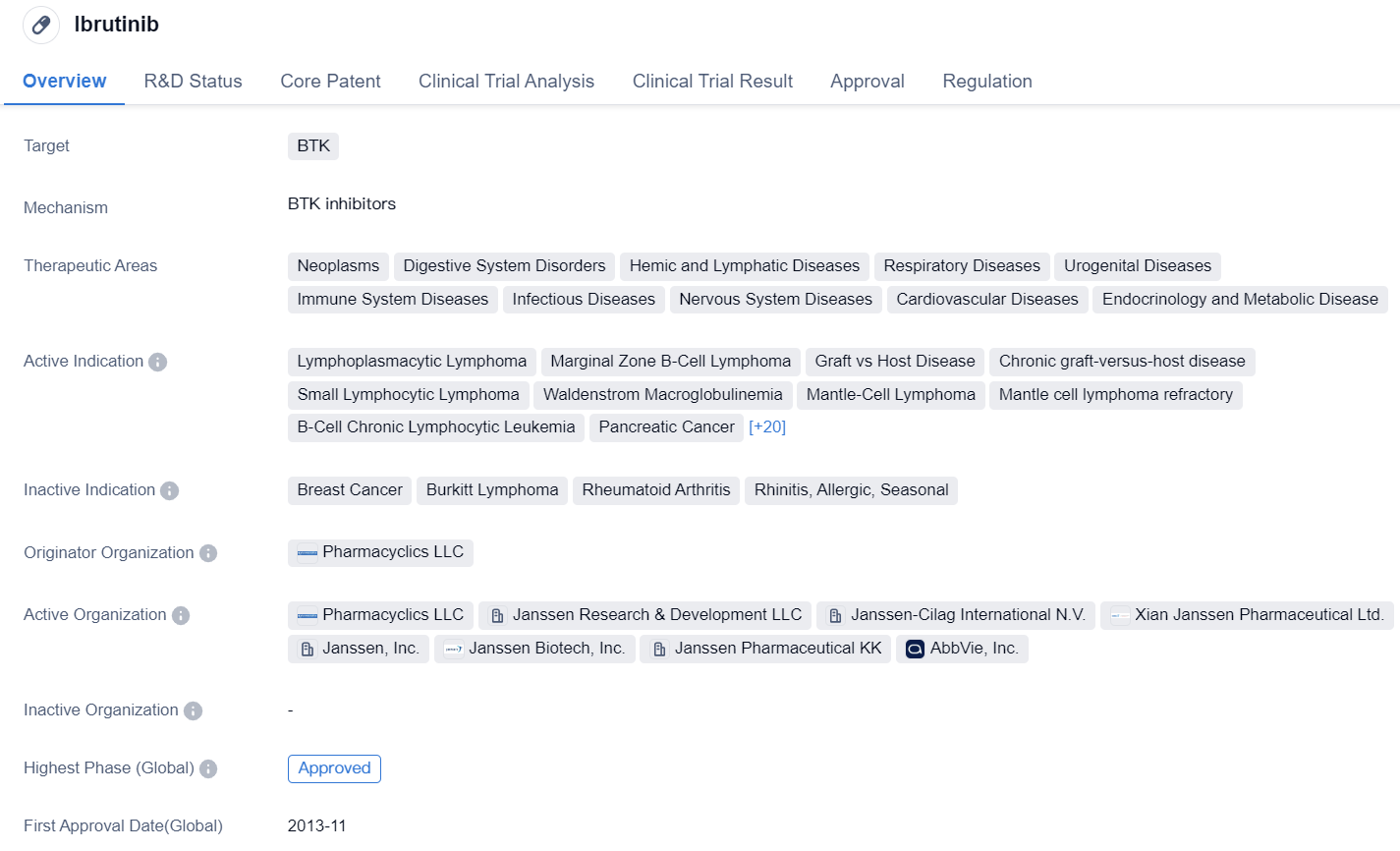
Ibrutinib is a small molecule drug that targets BTK. It has been approved for various therapeutic areas including neoplasms, digestive system disorders, hemic and lymphatic diseases, respiratory diseases, urogenital diseases, immune system diseases, infectious diseases, nervous system diseases, cardiovascular diseases, and endocrinology and metabolic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating several indications, including lymphoplasmacytic lymphoma, marginal zone B-cell lymphoma, graft vs host disease, chronic graft-versus-host disease, small lymphocytic lymphoma, Waldenstrom macroglobulinemia, mantle-cell lymphoma, mantle cell lymphoma refractory, B-cell chronic lymphocytic leukemia, pancreatic cancer, lymphoma, non-Hodgkin lymphoma, follicular lymphoma, diffuse large B-cell lymphoma, primary central nervous system lymphoma, COVID-19, SARS-CoV-2 acute respiratory disease, T-cell prolymphocytic leukemia, squamous cell carcinoma, B-cell lymphoma, central nervous system neoplasms, acute myeloid leukemia, multiple myeloma, renal cell carcinoma, urogenital neoplasms, gastrointestinal neoplasms, non-small cell lung cancer, myelodysplastic syndromes, neoplasms, and colorectal cancer.
The drug was originated by Pharmacyclics LLC and has received approvals in both the global and Chinese markets. It obtained its first approval in the United States in November 2013. Ibrutinib has undergone various regulatory processes, including priority review, accelerated approval, fast track designation, orphan drug status, breakthrough therapy designation, and special review projects.
In summary, Ibrutinib is a small molecule drug that targets BTK and has been approved for a wide range of therapeutic areas. It has shown efficacy in treating multiple indications, including various types of lymphomas, leukemias, solid tumors, and viral infections such as COVID-19. The drug was first approved in the United States in 2013 and has received regulatory designations to expedite its development and approval process.
Second Generation BTK Inhibitors:Acalabrutinib
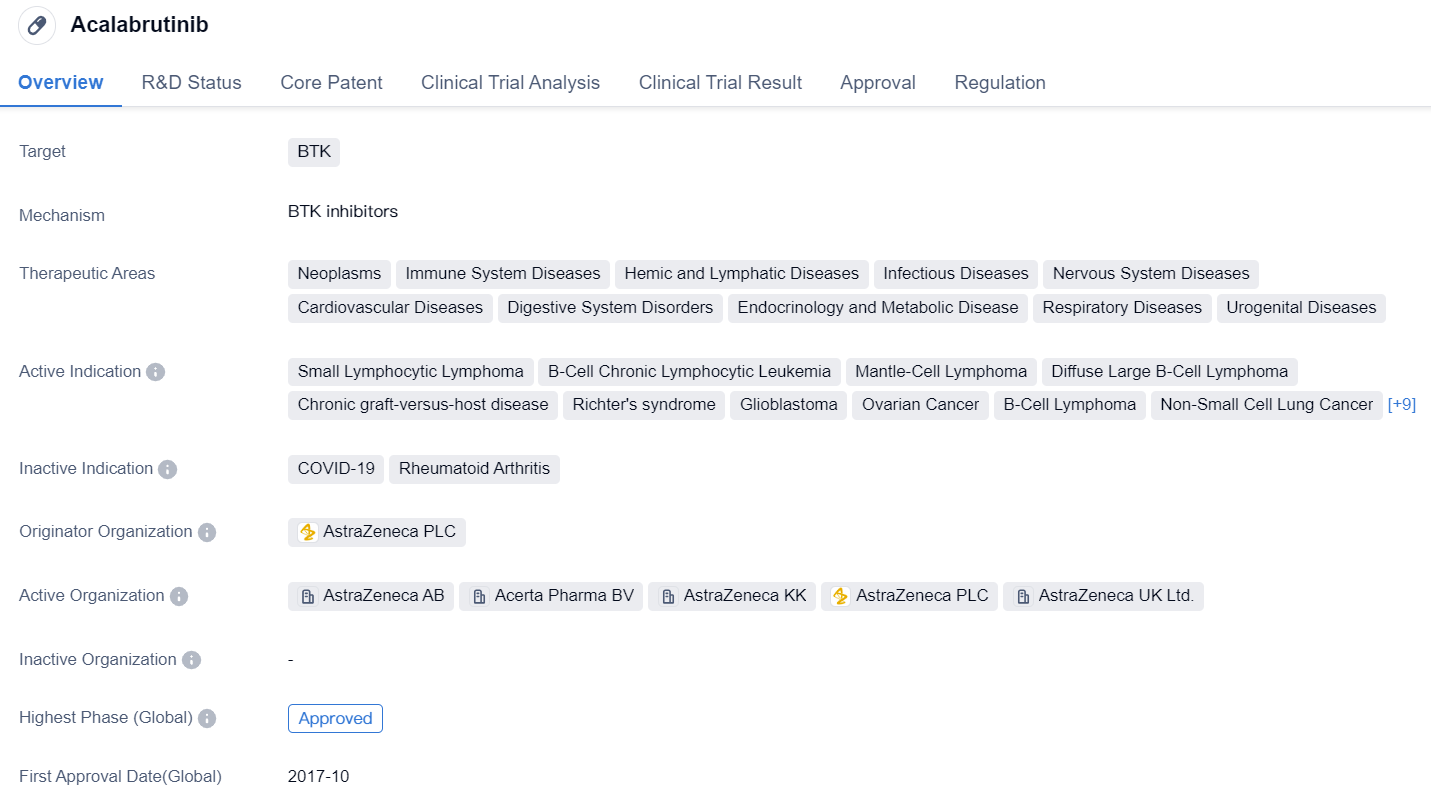
Acalabrutinib is a small molecule drug that targets BTK. It has been approved for various therapeutic areas including neoplasms, immune system diseases, hemic and lymphatic diseases, infectious diseases, nervous system diseases, cardiovascular diseases, digestive system disorders, endocrinology and metabolic disease, respiratory diseases, and urogenital diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating small lymphocytic lymphoma, B-cell chronic lymphocytic leukemia, mantle-cell lymphoma, diffuse large B-cell lymphoma, chronic graft-versus-host disease, Richter's syndrome, glioblastoma, ovarian cancer, B-cell lymphoma, non-small cell lung cancer, head and neck neoplasms, bladder cancer, pancreatic cancer, Burkitt lymphoma, multiple myeloma, Waldenstrom macroglobulinemia, prolymphocytic leukemia, follicular lymphoma, and B-cell prolymphocytic leukemia.
Acalabrutinib was developed by AstraZeneca PLC and has received approval in multiple countries, including the United States. Its highest phase of development is approved, indicating that it has successfully completed clinical trials and demonstrated safety and efficacy. The drug has also received priority review, conditional marketing approval, accelerated approval, orphan drug designation, and breakthrough therapy designation. These regulatory designations highlight the potential of acalabrutinib to address unmet medical needs and expedite its development and availability to patients.
The approval of acalabrutinib in various therapeutic areas underscores its versatility and potential to treat a wide range of diseases. Its mechanism of action, targeting BTK, suggests that it may be particularly effective in diseases involving abnormal B-cell signaling, such as lymphomas and leukemias. Additionally, its approval for neoplasms and various other disease areas indicates its potential to modulate the immune system and impact multiple disease pathways.
Overall, acalabrutinib represents a significant advancement in the field of biomedicine, offering a targeted therapeutic option for patients with various diseases. Its approval in multiple countries and regulatory designations further validate its potential and highlight the importance of continued research and development in the pharmaceutical industry.
Third Generation BTK Inhibitor:Pirtobrutinib
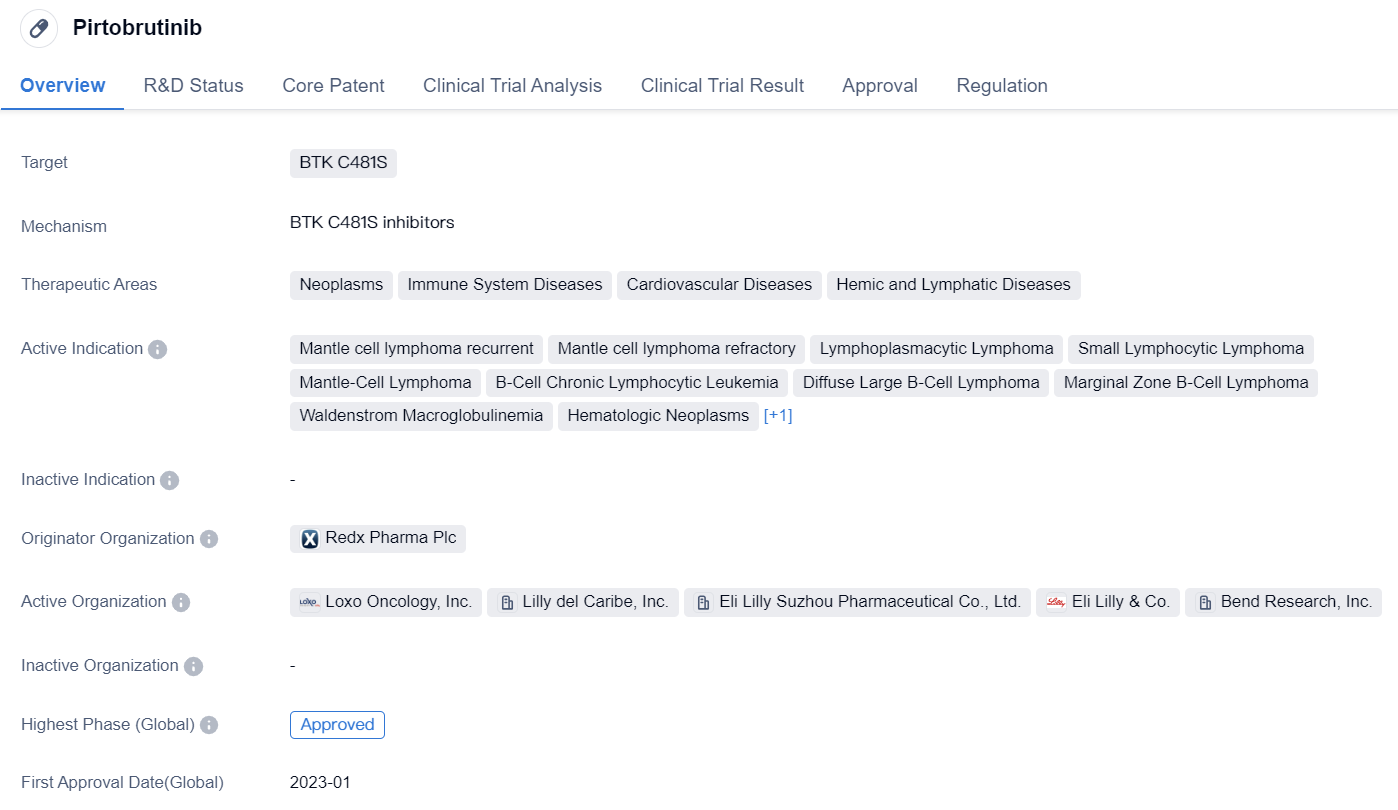
Pirtobrutinib is a small molecule drug that targets BTK C481S. It falls under the therapeutic areas of neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases. The drug is indicated for various conditions including mantle cell lymphoma recurrent, mantle cell lymphoma refractory, lymphoplasmacytic lymphoma, small lymphocytic lymphoma, mantle-cell lymphoma, B-cell chronic lymphocytic leukemia, diffuse large B-cell lymphoma, marginal zone B-cell lymphoma, Waldenstrom macroglobulinemia, hematologic neoplasms, and multiple myeloma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Pirtobrutinib is Redx Pharma Plc. The drug has reached the highest phase of approval globally, indicating that it has successfully completed clinical trials and has been approved for use. However, in China, it is currently in phase 3 of development, suggesting that it is still undergoing clinical trials in that country.
Pirtobrutinib received its first approval in the United States in January 2023. The drug has undergone priority review and accelerated approval, indicating that it has demonstrated significant benefits over existing treatments and has the potential to address unmet medical needs.
Based on the available information, Pirtobrutinib appears to be a promising drug in the field of biomedicine. Its approval for various indications related to lymphomas and other hematologic neoplasms suggests that it may have a broad therapeutic potential. The fact that it specifically targets BTK C481S, a protein associated with certain cancers and immune system disorders, further highlights its potential efficacy in treating these conditions.
The approval of Pirtobrutinib in the United States and its ongoing phase 3 development in China indicate that it has gained recognition and interest from regulatory authorities in these countries. This suggests that the drug may have significant market potential and could potentially become a valuable treatment option for patients in need.
In conclusion, Pirtobrutinib is a small molecule drug developed by Redx Pharma Plc that targets BTK C481S. It has received approval for various indications related to lymphomas and hematologic neoplasms, and its first approval was in the United States in January 2023. The drug has undergone priority review and accelerated approval, indicating its potential to address unmet medical needs. With ongoing phase 3 development in China, Pirtobrutinib shows promise as a valuable treatment option in the field of biomedicine.